-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Microbiology Research
p-ISSN: 2166-5885 e-ISSN: 2166-5931
2014; 4(2): 72-77
doi:10.5923/j.microbiology.20140402.05
Can Nigeria Sustain the Fight against Drug Resistant Mycobacterium tuberculosis?
Adegboyega Taofeek Tope1, Thomas Benjamin T.2, Agu Georgia C.3, Abiodun Abigail T.4
1Department of Biological Sciences, Faculty of Pure & Applied Sciences, Southwestern University, Okun-Owa, Ijebu-Ode, Ogun State, Nigeria
2Department of Cell Biology and Genetics, University of Lagos, Lagos State, Nigeria
3Department of Microbiology, Olabisi Onabanjo University, Ago-Iwoye, Ogun State, Nigeria
4National Tuberculosis Reference Laboratory, Nigerian Institute of Medical Research, Yaba, Lagos State, Nigeria
Correspondence to: Adegboyega Taofeek Tope, Department of Biological Sciences, Faculty of Pure & Applied Sciences, Southwestern University, Okun-Owa, Ijebu-Ode, Ogun State, Nigeria.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
Nigeria currently ranks 10th among the 22 high-burden TB countries in the world. The emergence of drug resistant TB and relatively high burden of HIV/AIDS have impacted negatively on TB control and prevention efforts. From 1982 in Lagos State for example, microscopy and culture back-up services were first provided to chest clinics to enhance case detection and reduce misdiagnosis. For the period under review, research studies to examine the possible impact of the erratic nature of TB care on diagnosis, clinical management and drug resistance was conducted. In all the efforts made, it was proffered that the problem of acquired resistance TB in Nigeria can be minimized with the introduction of affordable modalities for better supervision of drug administration and an adoption of a nation-wide level of effective short-course treatment. In achieving this, there must be improved financial commitment coupled with strong political will on the part of the Government and supporting donor agencies. This review is aimed at evaluating the treatment challenges of mycobacterial resistance in Nigeria. Drugs and TB care in the country is without any cost to the citizens. At present, there are improved and newer rapid molecular diagnostic techniques. The emerging challenge will therefore be how Nigeria will sustain the tempo and remain in the front burner of TB-Free Country in the future.
Keywords: Nigeria, Sustain, Fight, MDR-TB
Cite this paper: Adegboyega Taofeek Tope, Thomas Benjamin T., Agu Georgia C., Abiodun Abigail T., Can Nigeria Sustain the Fight against Drug Resistant Mycobacterium tuberculosis?, Journal of Microbiology Research, Vol. 4 No. 2, 2014, pp. 72-77. doi: 10.5923/j.microbiology.20140402.05.
Article Outline
1. Introduction
- Even though TB control and prevention efforts in Nigeria have progressed well over the last two decades of the introduction of the Directly Observed Therapy Short Course (DOTS) Strategy, it still constitutes a public health challenge. Tuberculosis (TB) is an infectious disease caused by Mycobacterium tuberculosis. It is transmitted by the inhalation of infective droplets from persons with pulmonary tuberculosis through coughing, sneezing, talking or spitting. The infected person suffers severe inflammation of the lungs and tissues necrosis which affects pulmonary functions. The World Health Organization (WHO) declared TB a global emergency in 1993 and it remains one of the world’s causes of illness and death.According to WHO (2011), Nigeria now ranks 10th among the 22 high burden countries for TB in the world. The emergence of drug resistant TB and relatively high burden of HIV/AIDS have impacted negatively on TB control and prevention efforts. In a review of the factors that contribute to an individual’s risk of exposure to the disease, the WHO (2009) listed factors such as the concentration of droplet nuclei in contaminated air, the duration of breathing in air and level of immunity of the individual. In addition, other factors include improper diagnosis and treatment, poor adherence to treatment and the presence of multi-resistant TB. Furthermore, the HIV/AIDS pandemic has also contributed to the deterioration of the infectious disease.If a person is infected with the bacteria, he/she can live a normal life without the development of the disease condition. However, in a patient with low immune system especially in HIV/AIDS infection, the infection will proceed to TB disease. In addition, some socioeconomic factors such as poverty, poor nutrition, overcrowding, substandard living and interaction with other diseases can increase risks to TB infection. Furthermore, high rates of re-infection can also increase morbidity and mortality in persons infected (WHO, 1996).One third of the world’s population, that is about two billion people carry the Mycobacterium tuberculosis. More than nine million of these become sick each year with active TB that can spread to others. The disease affects people in resource-poor countries particularly in Africa and Asia. It poses significant challenges to these economies as it primarily affects people during their most productive years. It is estimated that more than 90% of new TB cases and death occur in developing countries (Tuberculosis Fact Sheet, 2012).Drug treatment is very effective on the condition of strict patient’s adherence which may at times be difficult and prolonged. However, as a result of complexity in taking the drugs, it has been observed that patients do not adhere strictly to the instructions on the use of their medication. Hence, treatment failure, relapse and the development of drug-resistant TB strains do occur in patients who observe their treatment in an irregular and unreliable way (WHO, 2005).Due to patient’s non-adherence attitude towards their medication, continued increase death rate, the World Health Organization (WHO) introduced the Directly Observed Therapy Short Course (DOTS) Strategy. The DOTS strategy is a minimum of six months chemotherapy. It begins from registration of patients detected, followed by standardized multi-drug treatment with a secure supply of high quality anti-TB drugs for all patients in treatment.
2. Background to the Emergence of Drug Resistance in TB
- Holts et al., 2006 defined Multidrug resistance (MDR) TB as disease condition caused by strains of Mycobacterium tuberculosis that are resistant to both Rifampicin and Isoniazid with or without resistance to other TB drugs. It has been observed that treatment of MDR-TB is usually lengthy or prolonged and has the potential for the development of resistance to second line drugs.Extensive drug resistance (XDR) TB was first noted in the late 1980s and 1990s, and in 2004, the World Health Organization (WHO) and the United States Centres for Disease Control and Prevention (US CDC) reported in a survey of selected National Reference Laboratories that 20% of the strains tested were MDR-TB and that 10% of these were XDR-TB. The survey showed that rates of XDR-TB varied across the globe, with Asian and Eastern European Countries showing the highest rates. (CDC, 2006)However in 2006, the WHO defined XDR-TB as MDR-TB with additional resistance to any fluoroquinolone (FQ) and to at least one of the three injectable second-line drugs used in treatment [Capreomycin, Kanamycin or Amikacin] (WHO, 2006).
3. MDR-TB in Nigeria
- A pilot study conducted in Abuja by Lawson et al (2010) confirms that there is a high prevalence of multi-drug resistance in Nigeria. The study describes the presence of MDR-TB among new patients by using an automated culture system.Individualized Isoniazid and Streptomycin resistance had been reported in Nigeria in 7 and 2% of new patients with TB in the 1970s (Fawcett and Watkins, 1976). Subsequent unpublished studies using Lowenstein-Jensen (LJ) media and proportion drug susceptibility testing (DST) methods revealed that there was a rising trend in drug resistance.For instance, Kolo reported in 1991 that 19, 13 and 29% of newly diagnosed patients with TB in Zaria had Isoniazid (INH), Streptomycin (SM) and Pyrazinamide (PZA) resistance (Kolo, 1991). Resistance to these drugs was reported to have increased to 29, 14 and 42% by 2006 (Abdullah, 2006). This suggests that though MDR-TB may be poorly documented, it is likely to have been present among newly diagnosed patients in Nigeria for some time. The WHO (2011) reported that Nigeria has an estimated MDR-TB rate of 2.2% and 9.4% among new and re-treatment TB cases, respectively, and is therefore ranked 15th among the 27 High Burden Countries for MDR-TB. Nigeria is among the 4 African countries with the highest burden of drug-resistant TB. Cumulatively, only 142 confirmed MDR-TB cases out of the estimated 9,000-13,000 cases have been notified to the National TB and Leprosy Control Programme NTBLCP) between 2006 and 2011. (WHO, 2011 retrieved onlinehttp://www.afro.who.int/pt/nigeria/press-materials/item/4393-who-nigeria-support-for-rapid-scaling-up-of-programmatic-management-of-drug-resistant-tubercloisis-in-nigeria.html)Considering the above, there is need for good clinical management practices and a large country wide DST survey. In the same instance, it is likely that MDR-TB emerged in Nigeria in the 1990s as Idigbe et al (1992) reported that 56% of the strains recovered from 96 patients not responding to anti-TB treatment were resistant to one or more of the drugs used with 38% being resistant to Isoniazid (INH), only 2% was resistant to Rifampicin (RIF) at that time (Idigbe, et al., 1992). Based on the 2006 Annual Report of the National TB and Leprosy Control Program (NTBLCP), there is need for more concerted efforts at addressing TB control measures. Currently, the NTBLCP recommends treatment of newly diagnosed TB patients, with rifampicin, isoniazid, pyrazinamide, and ethambutol or streptomycin for 2 months, followed by isoniazid and rifampicin for 4 months or isoniazid and ethambutol for 6 months to reduce rifampicin interaction with the drugs used for the treatment of the human immunodeficiency virus (HIV) infection.
4. TB and HIV in Nigeria
- Idigbe, et al., (1998) in their study, did not find any link between TB and HIV infection. Presently, the Abuja pilot study by Lawson et al (2010) observed a high co-infection with TB. This restates the need to strengthen the linkage between TB and HIV services in Nigeria.The TB burden is compounded by a high prevalence of Human Immunodeficiency Virus (HIV) in the country which currently stands at 4.1% in the general population.The prevalence of HIV among TB patients increased from increased from 2.2% in 1991 to 25% in 2010. This shows that the TB situation in Nigeria is HIV-driven. (Tuberculosis Fact Sheet, 2012)The proportion of TB patients tested for HIV was 79% in 2010 with a 25% TB-HIV co-infection rate. 59% of these patients were on cotrimoxazole (CPT) prophylaxis and 1.8% provided with isoniazid (IPT) prophylaxis. Furthermore, the proportion of TB/HIV co-infected patients on anti-retroviral (ARV) therapy was 33% in 2010. In 2010 alone, the proportion of HIV-registered cases screened for TB was 57%. The proportion of HIV cases that developed TB was 4% in 2010 and 3% in 2011 (Tuberculosis Fact Sheet, 2012).
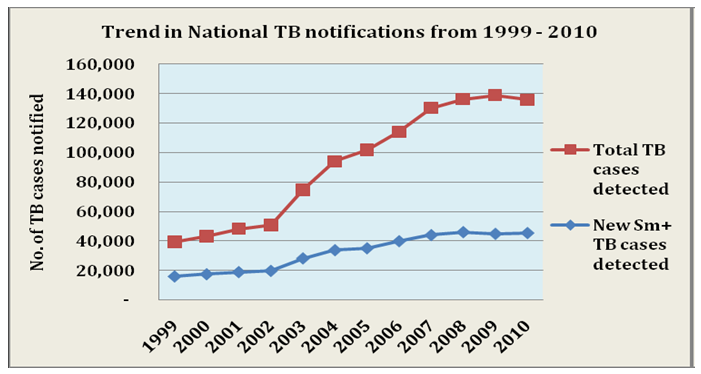 | Figure 1. Trend in National TB notifications from 1999-2010. Source: Nigeria Tuberculosis Fact Sheet, 2012 retrieved from http://nigeria.usembassy.gov |
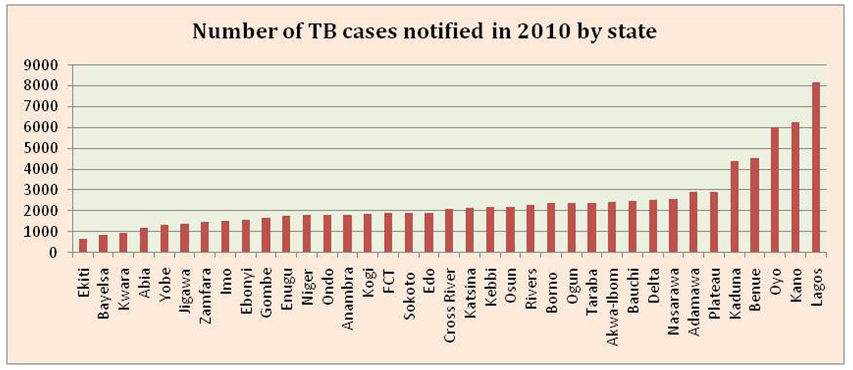 | Figure 2. TB by state notified in 2010. Source: Nigeria Tuberculosis Fact Sheet, 2012 retrieved from http://nigeria.usembassy.gov |
5. AGE and Sex Distribution
- TB is noted for affecting the most productive age group with the 25-34 age group accounting for 33.6% (15,303) of the smear positive cases registered in 2010. (Tuberculosis Fact Sheet, 2012). The HIV status in relation to age was not considered in the report highlighted.
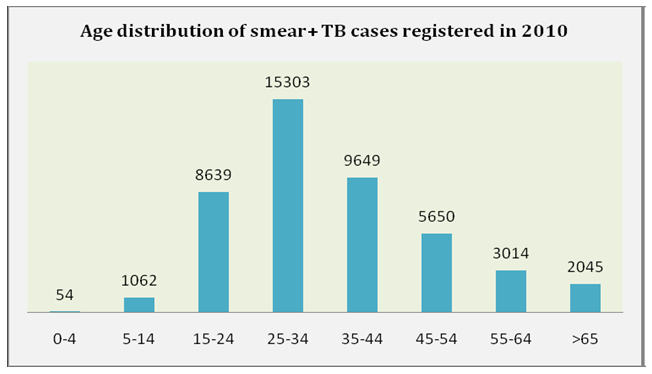 | Figure 3. Age distribution of Smear Positive TB cases registered in 2010. Source: Nigeria Tuberculosis Fact Sheet, 2012 retrieved from http://nigeria.usembassy.gov |
6. Challenges and Limitations to TB Treatment in Nigeria
- Despite the immense wealth in the country, TB treatment and management in Nigeria has been bedevilled with many challenges which include; poor health infrastructure, poor patients referral system and lack of effective data system/archival system. Others are lack of efficient collaboration between TB and HIV program, poor procurement and supply management system if drugs and other consumables, reduced coverage of IPT, ARV and CPT for TB/HIV co-infected individuals, lack of ownership of TB Control Programs (response is majorly donor driven) and corruption which have eaten deep into the fabrics of the country.
7. The World Health Organization’s (WHO) Support to Nigeria’s Quest for Rapid Scaling-Up of Programmatic Management of Drug-Resistant Tuberculosis (PMDT)
- In response to the threat of Drug Resistant-TB, the Federal Ministry of Health (FMoH) Nigeria in collaboration with WHO and other partners have put in place structures for the initiation and expansion of the programmatic management of drug-resistant TB in the country with a view of effectively controlling its spread and reduce transmission (WHO, 2011 retrieved onlinehttp://www.afro.who.int/pt/nigeria/press-materials/item/4393-who-nigeria-support-for-rapid-scaling-up-of-programmatic-management-of-drug-resistant-tubercloisis-in-nigeria.html)On the 23rd May, 2013, the WHO supported the country with items such as 36 ILED Microscopes, reagents and consumables worth $126,000.00 through the Federal Ministry of Health. Presently in Nigeria, there is a functional National DR-TB Committee and policy guidelines on PMDT (programmatic management of drug-resistant tuberculosis) are in place. The enrolment of MDR-TB for treatment with second-line anti-TB drugs started in July 2010 at the only functional MDR-TB treatment centre at University College Hospital, Ibadan. (WHO, 2012)The pattern of care involves an initial hospitalization until sputum culture conversion followed by ambulatory period of treatment in the nearest DOTS facility. The NTBLCP is now set to expand the implementation of PMDT.Milestones in the Implementation PMDT: As a major technical partner of the NTBLCP, WHO has continued to play key roles in many critical aspects of PMDT implementation in Nigeria.Funding and Policy Issues: Leveraging on Global Fund for AIDS, Tuberculosis and Malaria (GFATM) grant to support the National scale plan for the programmatic management of drug-resistant TB (PMDT). Grant was signed in July 2011 between the Global Fund and the Institute of Human Virology of Nigeria (IHVN) as the Principal Recipient (PR); development and finalization of the National Guidelines for the Clinical and Control of Drug Resistant TB in Nigeria. The NTBLCP leveraged funds from WHO to develop and finalize DR-TB training guidelines to ensure the standardization of training for all facilities and field health workers providing DR-TB management services.Infrastructural Development: With support from KNCV/TBCARE 1, the Gene Xpert MTB/RIF technology has been deployed to 9 facilities across the country. The facilities are; National TB Reference Laboratories in Lagos and Zaria, Chest Hospital Jericho-Ibadan, Infectious Disease Hospital (IDH) Kano, Specialist Hospital Gombe, Mile 4 Mission Hospital, Abakaliki, Central Hospital Benin-City and Zankli Medical Centre Abuja. This is already improving access to rapid and early diagnosis. (WHO, 2012)The WHO participated actively in the planning meetings for the selection and assessment of the facilities for the deployment of the Gene Xpert machines. The BSL 3 Laboratory at the National Reference Laboratory/Nigerian Institute of Medical Research (NRL/NIMR) Lagos is operational as well as the BSL 2 section is fully functional. The two NRLs, in Lagos and Zaria have capacities for solid/liquid culture and DST as well as for line probe assay (LPA) and Gene Xpert MTB/RIF. Five additional DR-TB Treatment Centres are now ready and have commenced the enrolment of confirmed MDR-TB patients for treatment on Second Line Anti-TB Drugs (SLDs) in the first week of March 2012. These facilities are located at Dr Lawrence Henshaw Memorial Infectious Disease Hospital, Calabar (14-bed capacity); Lagos Mainland Hospital (40 bed capacity); Jericho Chest Hospital, Ibadan (25 bed capacity); National TB and Leprosy Training Centre, Zaria (40 bed capacity) and Infectious Disease Hospital, Kano (25 bed capacity) (WHO, 2011 retrieved online http://www.afro.who.int/pt/nigeria/press-materials/item/4393-who-nigeria-support-for-rapid-scaling-up-of-programmatic-management-of-drug-resistant-tubercloisis-in-nigeria.html).Human Resource Development: The WHO provided technical support for the training on DR-TB management for 27 medical and health workers from three of the newly established DR-TB treatment Centres at Calabar, Ibadan and Lagos. Plans to train the medical and health workers from the other centres at Kano and Zaria have been concluded. Plans are also in place to train the programme staff at all levels on PMDT.These training activities will facilitate the effective and systematic decentralization of PMDT in the country. NTBLCP has also trained a critical pool of core trainers the M & E (Monitoring and Evaluation) aspect of DR-TB.Drugs Logistics and Patient Treatment: So far two cohorts of 61 MDR-TB patients (23 in 2010 and 38 in 2011) have been enrolled on treatment (WHO, 2011 retrieved onlinehttp://www.afro.who.int/pt/nigeria/press-materials/item/4393-who-nigeria-support-for-rapid-scaling-up-of-programmatic-management-of-drug-resistant-tubercloisis-in-nigeria.html). The interim treatment outcome so far shows that 41 have converted and now on ambulatory treatment (after the initial hospital treatment); 11 are still on admission while nine have died. The NTBLCP took delivery of SLDs for 110 MDR-TB patients. The WHO has continued to play important role in strengthening patient management and in the clearance of the drugs on behalf of the NTBLCP.Programme Monitoring and Routine Surveillance: Working in collaboration with the NTBLCP and other partners, the WHO supported the adaptation of the recording -and reporting formats for DR-TB, which have been printed and available for use in the field. Technical support was also provided in the development and establishment of routine surveillance system for DR-TB. As part of the scale up plan, a system of routine culture/DST for all identified re-treatment cases is now in place. From both the conceptual and operational points of view, routine culture/ DST will be expanded to other risk groups over the next few years as more resources become available. NTBLCP in collaboration with MSH leading other partners including WHO have customized the E-TB manager for documentation and monitoring purposesOperational Research: Through funding support from WHO/Geneva, the NTBLCP in collaboration with other partners conducted an operational research on operationalising Community-based DR-TB care in the country (WHO, 2011 retrieved onlinehttp://www.afro.who.int/pt/nigeria/press-materials/item/4393-who-nigeria-support-for-rapid-scaling-up-of-programmatic-management-of-drug-resistant-tubercloisis-in-nigeria.html). The findings are insightful and provide operational contexts to guide the development of models for community-based DR-TB control and prevention in the country (WHO, 2012)
8. Recommendations
- 1. More efforts should be geared towards knowing the actual prevalence of MDR-TB cases in each of the 36 states and the Federal Capital Territory (FCT) in Nigeria. 2. Referral systems for drug resistant patients should be more enhanced. The continued expansion of the DOTS strategy throughout the country should be pursued more vigorously to prevent increases in drug resistance. Operational interventions such as screening and early diagnosis, contact tracing especially of first-degree relatives with MDRTB should be organized.3. Adequate measures should be put in place to provide second-line drugs, supervision of drug distribution and compliance, enforcement of DOTS protocols and continued training of all personnel in TB management and care.4. Further studies are required to obtain and derive more precise estimates of drug resistance in defined groups. There are very few studies and limited data on DR-TB published in literature. 5. Laboratory capacity in the country should be improved upon by the provision of faster, automated culture and DST facilities or automated polymerase chain reaction (PCR) technologies in health care facilities. 6. Future studies should be carried out to investigate if extensively drug resistant TB (XDR-TB) strains are also affecting newly diagnosed patients with TB. 7. In the interim, there is need to establish more national reference laboratories to complement the existing four laboratories providing services on drug resistance. 8. Linkages between our laboratories and existing supra-national reference laboratories (SRL) overseas should be made to ensure quality assurance and technical expertise. 9. The Federal Government, the Federal Ministry of Health and her allied partners should not give up efforts to help sustain the tempo on DR-TB in Nigeria. This is because Nigerians lack good maintenance as well as continuous improvement culture which allays fear that if the current tempo is not sustained, we may go back to a worse situation than where we were before now. 10. We therefore strongly suggest that if the above measures are implemented, it will go a long way in achieving the reduction by half of tuberculosis in Nigeria in line with the Millennium Development Goals (MDGs) before 2015.
9. Conclusions
- This review has established that drug resistant TB (mono drug resistant and MDR TB) is present in Nigeria. It has also been found that Nigeria has intensified efforts to increase programmatic management of drug resistant TB. Funding has been provided for effective case finding, treatment and monitoring of drug sensitive and resistant TB in Nigeria. This has led to provision of infrastructures and strengthening of capacities in diagnosis, treatment, monitoring and evaluation of interventions for drug resistant.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML