-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Microbiology Research
p-ISSN: 2166-5885 e-ISSN: 2166-5931
2014; 4(2): 29-42
doi:10.5923/j.microbiology.20140402.01
Enzymatic Studies and Mineralization Potential of Burkholderia cepacia and Corynebacterium kutscheri Isolated from Refinery Sludge
Ajao A. T. 1, Yakubu S. E. 2, Umoh V. J. 2, Ameh J. B. 2
1Department of Science Lab. Tech., Microbiology Unit, Institute of Applied Sciences, Kwara State Polytechnic, Ilorin, Nigeria
2Department of Microbiology, Ahmadu Bello University, Zaria, Nigeria
Correspondence to: Ajao A. T. , Department of Science Lab. Tech., Microbiology Unit, Institute of Applied Sciences, Kwara State Polytechnic, Ilorin, Nigeria.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Environmental pollution with petroleum hydrocarbons has been recognized as a serious concern worldwide causing ecological imbalance. Mineralization is essential for the conversion of petroleum hydrocarbon and hazardous pollutants into less toxic compounds, mostly water and carbon dioxide. In this study, refinery sludge samples from Kaduna Refining and Petrochemical Company were collected. These were analyzed by selective enrichment technique, resulted in the isolation of two bacterial species which are Burkholderia cepacia and Corynebacterium sp. Crude oil degradation was performed by the pure and mixed culture of the isolates incubated in the shake flask culture. Optical density, pH, Protein, Dehydrogenase and DHA activities were used as bioindicators for the degradation processes while CO2 production and GC-MS were used to analyze Mineralization potential. Dehydrogenase activities, Growth pattern, Protein estimation and FDA are strongly correlated with the rate of biodegradation therefore; these parameters can be used as indicators for aerobic biodegradation of crude oil while This research confirmed that Biodegradation of petroleum hydrocarbon generally relies upon the cooperation of more than a single bacterial species, and such cooperation that exists between the isolates is quorum sensing phenomenon. These findings have environmental implication towards developing a microbial consortium that could be exploited for cleaning oil polluted environment.
Keywords: Mineralization, Bioindicator, Crude oil, GC-MS, DHA, FDA
Cite this paper: Ajao A. T. , Yakubu S. E. , Umoh V. J. , Ameh J. B. , Enzymatic Studies and Mineralization Potential of Burkholderia cepacia and Corynebacterium kutscheri Isolated from Refinery Sludge, Journal of Microbiology Research, Vol. 4 No. 2, 2014, pp. 29-42. doi: 10.5923/j.microbiology.20140402.01.
Article Outline
1. Introduction
- The impacts of crude petroleum, prosperity and production operation on oil producing countries of the world have resulted in enormous pollution of air, water and particularly agricultural land for food production [1,2]. Crude oil pollutant is a recalcitrant molecule toxic to biotic factor and persists in environment for many years depending on the natural and quality of oil spilled [3,4].The removal of oil that has been accidentally or purposely spilled into the environment is therefore of great concern to the petroleum industry [23]. Previous investigations have shown that crude oil and other related organic pollutants can be degraded [5,6,7,8]. Hydrocarbon-degrading microorganisms are widely distributed in marine, freshwater, and soil ecosystems [9]. The ability to isolate high numbers of certain oil-degrading microorganisms from an environment is commonly taken as evidence that those organisms are active degraders of that environment [10].There is an extensive body of knowledge on mineralization or degradation of hydrocarbons by microorganisms [11, 12]. In a recent study, Ojo [13] reported the capability of native bacterial population to mineralize petroleum hydrocarbons in wastewater.Malik and Ahmed [14] reported that there is no single strain of bacteria with the metabolic capacity to degrade all the components found within crude oil. In nature, biodegradation of a crude oil typically involves a succession of species within the consortia of microbes present. A combination of bacterial strain with broad enzymatic capabilities is required for active extensive degradation of crude oil.It is the process of using microorganisms to convert petroleum hydrocarbon and hazardous pollutants into less toxic compounds mostly water and carbon dioxide, a process called mineralization. It is a natural process, microbes work to break down practically all hydrocarbon contaminants, in the natural environment. It transforms pollutions, and not changes them from one medium to another. Also, it degrades a wide range of different pollutions. Relative to other methods of pollutant removal bioremediation is of reduced risk and huger degree of safety, reduced labour and very cost effective [15].In many ecosystems there is already an adequate indigenous microbial community capable of extensive oil biodegradation, provided that environmental conditions are favorable for oil-degrading metabolic activity [16,17,18]. The ability of a biosurfactant to emulsify hydrocarbon water mixtures have been widely reported [19,20]. These emulsification properties have also been demonstrated to enhance hydrocarbon degradation in the environment; hence making them potentially useful tools for oil spill pollution control [21].A number of investigation which focus on solving the problems of degradation of oil reported in the scientific literatures are purely biotransformation or co-metabolism rather than mineralization which can result into the formation of intermediate products that are not only toxic but also may remain recalcitrant and persistent in the ecosystem [22]. The immense potential of microorganisms to mineralize petroleum hydrocarbon does not only depend on the ability to provide energy or cell biomass, catabolic enzymes but also on their metabolic activities [23]. Mineralization is a combined activities of microbial consortia that does not produce intermediates pollutants in the environment, the final products are CO2 and H2O [24]. The present work has the aim of investigating the mineralization potential and characterization of Hydrolytic enzymes involved in biodegradation of crude oil by Autochthonous Burkholderia cepacia and Corynebacterium kutscheri in refinery sludge.
2. Materials and Methods
2.1. Collection of Sample
- Refinery sludge was collected from under the storage tank in Kaduna Refining and Petrochemical Company (KRPC) Kaduna State, Nigeria using sterile trowel into polyethylene bags, stored at 4°C and kept in sterile containers. Analysis commenced immediately upon arrival in the department of Microbiology laboratory.
2.2. Isolation of Bacteria
- A selective enrichment technique was used for the isolation of hydrocarbonoclastic bacteria from Kaduna refinery sludge. Bushnell-Haas broth was used as enrichment media supplemented with 5%v/v of Bonny light crude oil. Specifically, 10g of refinery sludge was incubated on rotary shaker at room temperature in 195ml of Bushnell-Haas medium. It was incubated for two weeks in a rotary shaker at 120rpm. Aliquot of 0.1mililiter from the set up was plated on Plate count agar after two weeks and incubated at 26°C for 48hours. The colonies form was stored as stock cultures before used [25,26].
2.3. Identification of Bacteria
- The two isolates that survived 2weeks selective enrichment technique were subjected to Biochemical and Physiological Characterization. Identification of the isolates to the generic level was done following the scheme in Bergey’s Manual of Systematic Bacteriology [27]. This was finally confirmed using an automated test system Vitek Analytical Profile Index (API20E) BioMerieux, inc) A manufacturer’s specification for proper characterization and identification of the isolates was followed.
2.4. Mineralization Potential of Isolated Microorganisms
- The ability of the bacterial isolates to mineralize Bonny light crude oil as the only source of carbon and energy was determined by the method of [6,13,10,29] with some modifications. The mineralization assay was carried out as follows: Each of the bacterial isolates was tested for their ability to mineralise crude oil using a liquid mineral medium, Bushnell-Haas (BH) medium [30]. A single colony of the isolate was inoculated into 10 ml nutrient broth (Merck) and incubated at 30°C overnight at 160 rpm. The overnight culture was centrifuged for 10 min at 5000 rpm. The cell pellet was washed twice in phosphate buffer saline (pH 7.6) and re-suspended in BH medium until OD600 nm was equivalent to 1.0ml of bacterial inoculum (1=OD600 nm equivalent) was added to 250ml Erlenmeyer flasks containing 100 ml of liquid BH medium supplemented with 2% carbon source (Bonny light crude oil), pH of the medium was adjusted to 7.0. Pure and mixed culture of the cells was cultivated separately. The control flasks contained the entire nutrient and the oil but no bacterial culture. All the flasks were incubated at 30℃ for 18days on an orbital shaker at 120rpm. Assay was carried out in duplicates.Growth was monitored by testing optical density (OD600) at 600 nm after every 24hours. pH, Dehydrogenase Activities [31], Fluorescein Diacetate Analysis [32], Emulsification test [33]. CO2 Production [34, 25, 35], and Estimation of Total protein [36].
2.5. Gas Chromatographic Analysis
- Gas chromatographic analysis was made according to the methods of [37]. After the incubation period, 5ml of the cultures were extracted with 20ml volumes of n-hexane as a solvent by using separatory funnels to remove cellular material. The residues were transferred to tarred vials and the volume of each extract was adjusted to 100ml by adding further n-hexane. The vials were kept at 4°C until the gas chromatographic analysis was carried out. Uninoculated control was incubated in parallel to monitor abiotic losses of the substrate.
3. Results
- The isolation and identification of crude oil degrading bacteria in Kaduna Refinery Petrochemical Company (KRPC) from oil sludge were identified based on colonial morphology biochemical and physiological characteristics of the bacteria were carried out. Automated test system vitek API 20NE and API Coryne. (BioMerieux, inc Hazelwood, Mo, USA) was used for the confirmation and authentication of the genera belonging to Burkholderia cepacia and Corynebacterium kutscheri as shown in Table 1.
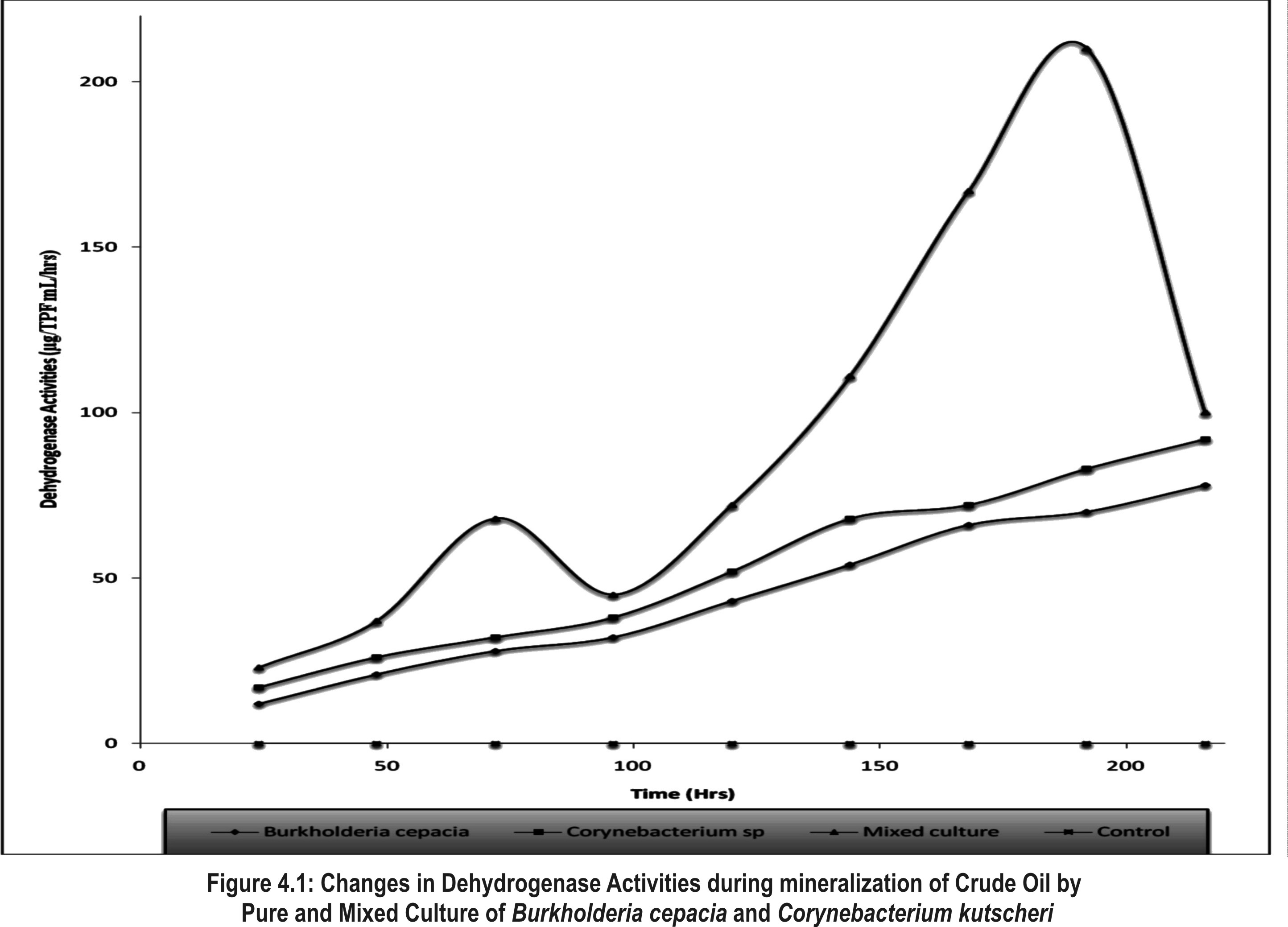 | Figure 1. Changes in Dehydrogenase Activities during mineralization of Crude Oil by Pure and Mixed Culture of Burkholderia Cepacia and Corynebacterium Kutscheri |
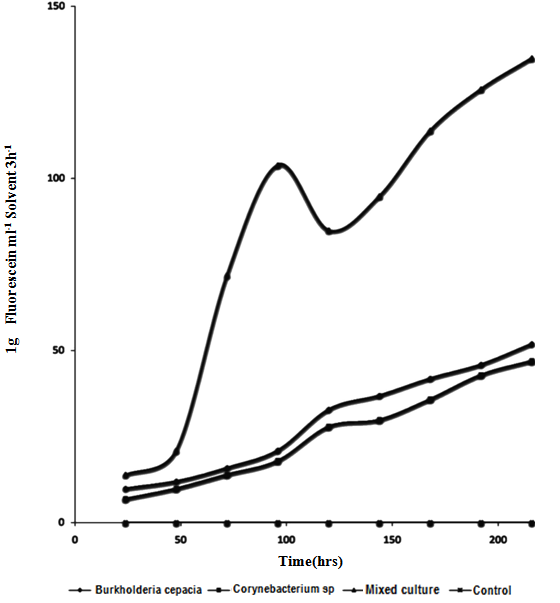 | Figure 2. Changes in Fingerprints Diacetate during mineralization of Crude Oil by Pure and Mixed Culture of Burkholderia Cepacia and Corynebacterium Kutscheri |
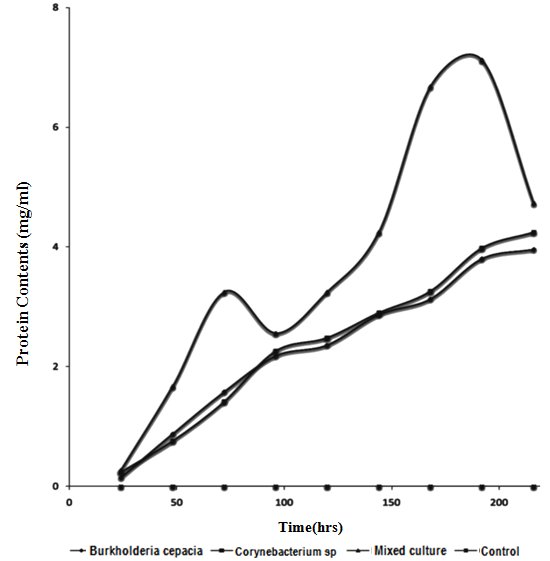 | Figure 3. Changes in Protein contents during mineralization of Crude Oil by Pure and Mixed Culture of Burkholderia Cepacia and Corynebacterium Kutscheri |
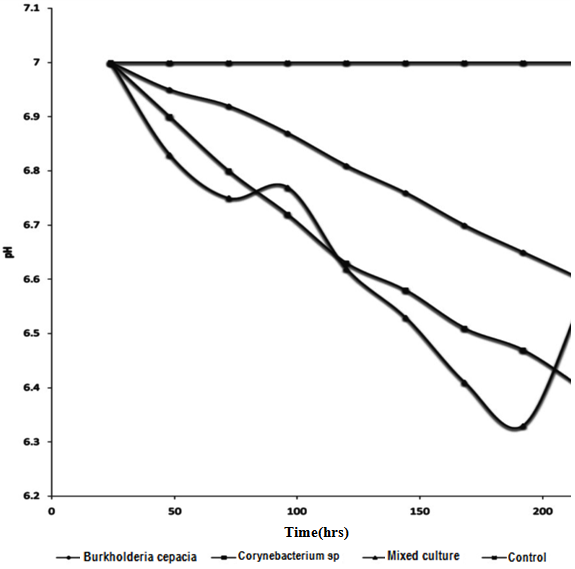 | Figure 4. Changes in PH during mineralization of Crude Oil by Pure and Mixed Culture of Burkholderia Cepacia and Corynebacterium Kutscheri |
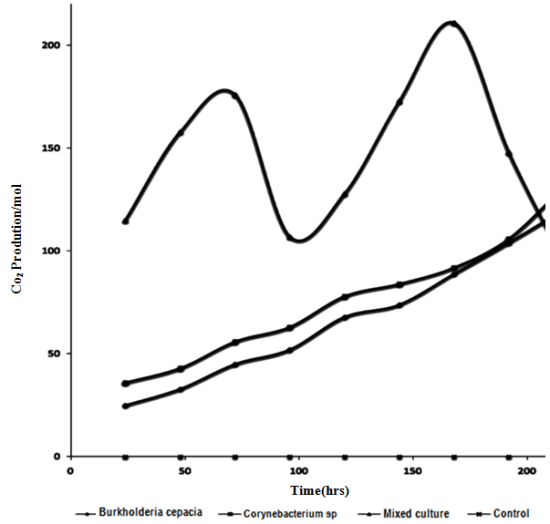 | Figure 5. Changes in CO2 production during mineralization of Crude Oil by Pure and Mixed Culture of Burkholderia Cepacia and Corynebacterium Kutscheri |
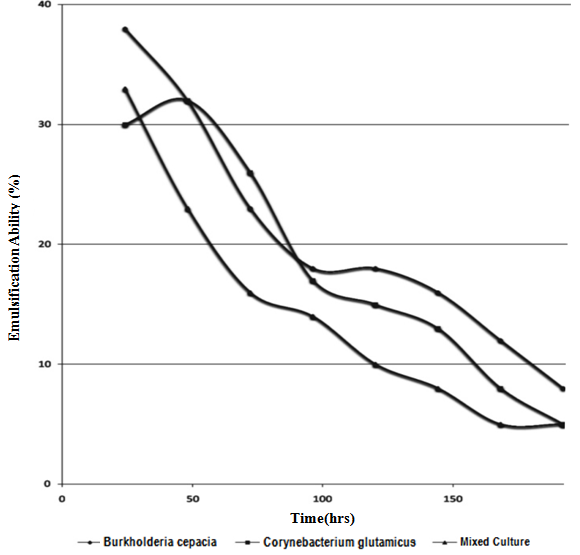 | Figure 6. Changes in Emulsification Ability of Pure and Mixed Culture of Burkholderia Cepacia and Corynebacterium Kutscheri |
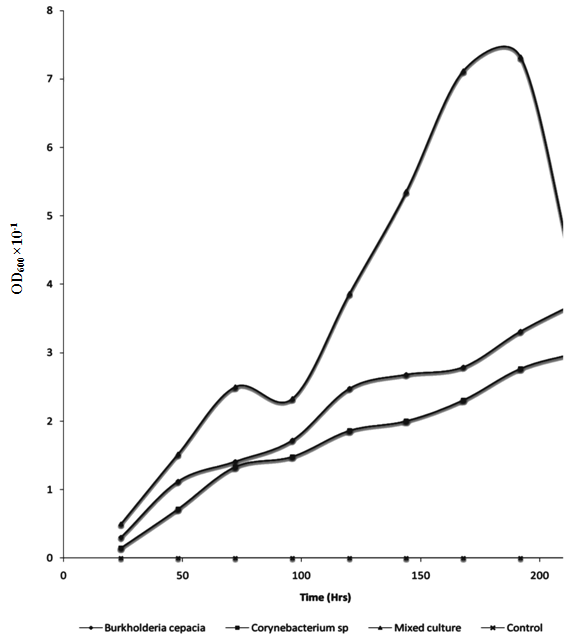 | Figure 7. Changes in Bacterial Growth during Mineralization of Crude Oil by Pure and Mixed Culture of Burkholderia Cepacia and Corynebacterium Kutscheri |
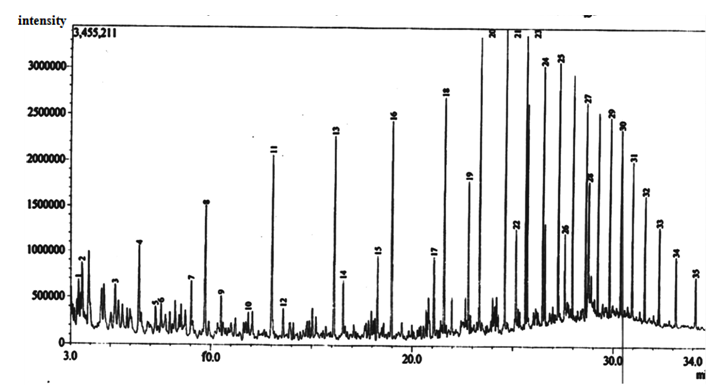 | Figure 8. Gas Chromatographic Fingerprints of Bonny Light Crude Oil recovered from Flasks Uninoculated with Bacterial Isolates (Control) |
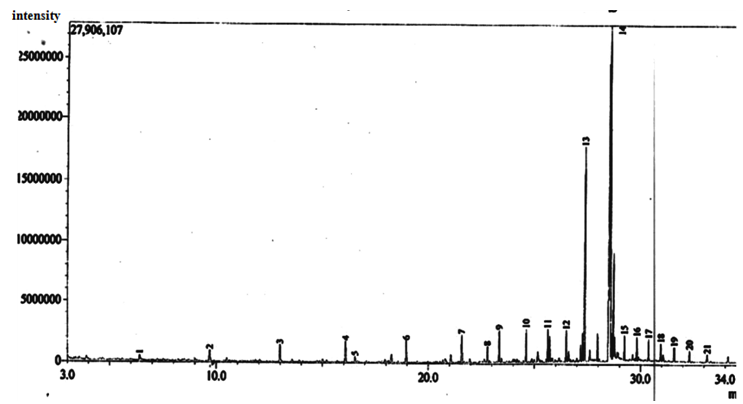
 | Figure 9. Gas Chromatographic Fingerprints of Bonny Light Crude Oil recovered from Flasks Inoculated with Burkholderia Cepacia after 5 days of Incubation |
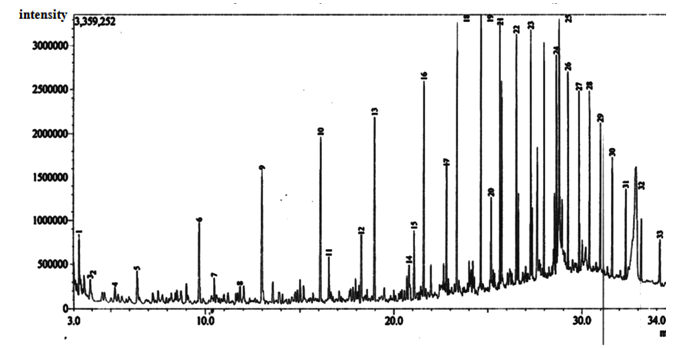
 | Figure 10. Gas Chromatographic Fingerprints of Bonny Light Crude Oil recovered from Flasks Inoculated with Burkholderia Cepacia after 10days of Incubation |
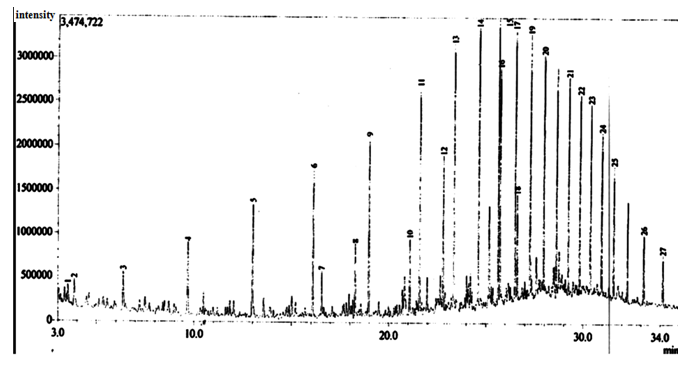
 | Figure 11. Gas Chromatographic Fingerprints of Bonny Light Crude Oil recovered from Flasks inoculated with Mixed Culture of Burkholderia Cepacia and Corynebacterium Kutscheri, after 5 days of Incubation |
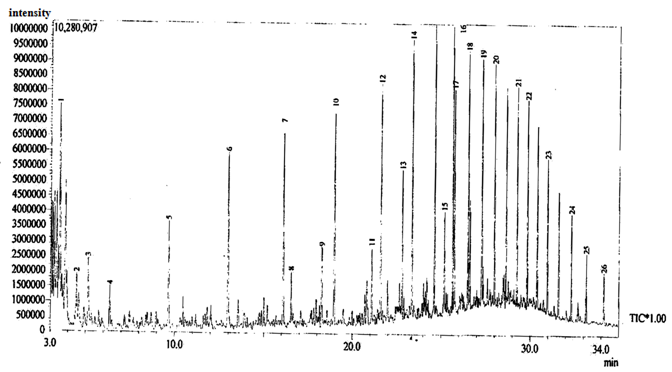 | Figure 12. Gas Chromatographic Fingerprints of Bonny Light Crude Oil recovered from Flasks Inoculated with Burkholderia Cepacia after 10 days of Incubation |
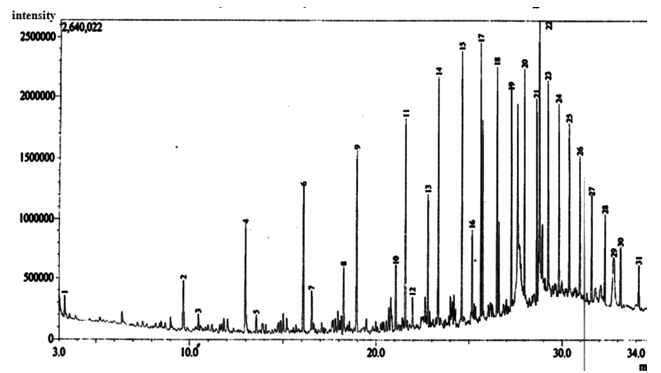 | Figure 13. Gas Chromatographic Fingerprints of Bonny Light Crude Oil recovered from Flasks Inoculated with Corynebacterium Kutscheri, after 10 days of Incubation |
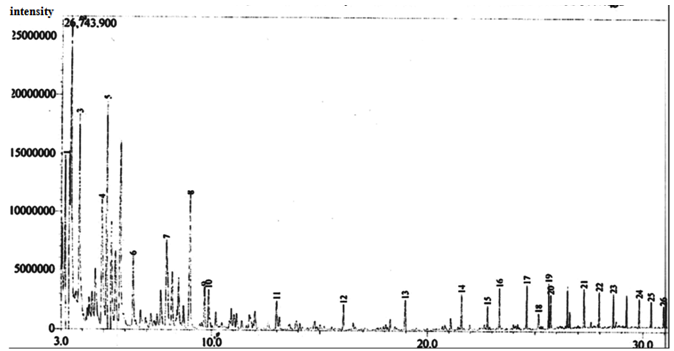 | Figure 14. Gas Chromatographic Fingerprints of Bonny Light Crude Oil recovered from Flasks Inoculated with Culture of Burkholderia Cepacia and Corynebacterium Kutscheri, after 10 days of Incubation |
4. Discussion
- The presence or ability of the two isolates to survive 5% concentration of crude oil during two weeks of enrichment technique is important which seemed to have led to the synthesis of enzymes involved in crude oil metabolism or changes in the genetic capacity of microbial species to maintain their ability to degrade crude oil [38]. This findings showed that enrichment technique is useful for the isolation of various culturable oil degrading bacteria from contaminated sites for making consortia use in bioremediation [40]. The mechanisms employed by the autochthonous microorganisms to achieve this feat includes synthesis of inducible enzymes, mutations such as single nucleotide change or DNA re-arrangement that results in degradation of the compound and acquisition of genetic information from closely related or phylogenetically distinct population within the hydrocarbon-challenged community [39]. The occurrence of Burkholderia cepacia and Corynebacterium kutscheri in oil sludge is in line with the work of [41] who reported the degradation of heavy crude oil (maya) by B. cepacia strain RQI. There are other reports of Burkhoderia sp RQ1 capable of remarkable growth on aromatic fractions of crude oil [42, 43, 44]. The biodegradation potentials of Corynebacteium sp have also been reported by [45, 46, 47]. The slight increase after 72hours of incubation of Dehydrogenase activity in the culture filtrate can be attributed to the repression and induction of both inducible and constitutive enzyme involved in the biodegradation due to the presence of utilizable hydrocarbon substrates, while dip drop in 96hours may be as a result of non availability of utilizable hydrocarbon substrates that led to co-metabolism and increase in enzymatic activities.As optical density and protein content increased dehydrogenase activity would also increase, this hypothesis is supported by the smaller increase in dehydrogenase which led to increase in bacteria growth and protein content this is in agreement with the work of [48] who reported that the extracellular enzymes of organisms are produced in response to their growth phases. Wang et. al., [49] reported that consumption of easily-available organic-C in the experimental medium may directly result into high content of biomass and dehydrogenase activity.A gradual increase in FDA observed in 96 hours can be attributed to high enzymatic activity, which may be as a result of the presence of non-degradable hydrocarbon compounds that led to decrease in both DHA and CO2 production. Such increase in FDA can result into co-metabolism leading bioconversion of the substrate without releasing energy. It is reported that FDA is closely related to total C, total N, and total P and other nutrient indices in experimental medium and that it has close correlation between conversion status of organic matter and overall microbial activity [32, 50]. Slight drop in 120hrs of incubation may be attributed to availability of utilizable hydrocarbon substrate as later increased progressively, such FDA production pattern not considered a representative measure of actual biodegradation because FDA did not decrease when all other parameters had decreased after mineralization, this is in agreement with work of Lee et al., [51].Protein content represents the total enzyme in the medium both inducible and constitutive enzymes. Sepahi et al., [52] reported that the protein estimation is an effective method for monitoring the microbial population in an experimental medium.It can be deduced that the growth dynamic of the isolate, dehydrogenase activities and protein contents are proportionate when there is availability of utilizable hydrocarbon compounds, therefore, the increase or decrease in protein concentration, Dehydrogenase activities and optical density during incubation period revealed that the isolates can utilize Bonny light crude oil as source of carbon and energy [45,52,53] also in their work, they showed that the same pattern of relationship existing between the protein content and microbial growth during mineralization of crude oil. The reduction in pH of the culture filtrate in the experimental flasks within the incubation period further confirmed chemical changes of the hydrocarbon substrates which must have been precipitated by microbial enzymes [9]. It has been reported that microbial degradation of hydrocarbon often leads to production of organic acids and other metabolic products [54,55]. Thus, the acids probably produced accounted for the reduction in the pH levels. This is important in view of the microbial survival and adaptation in terms of suppressing effect on the synthesis of enzymes involved in crude oil metabolism or by changes in the genetic capacity of microbial species to maintain their ability to degrade crude oil [38].Biosurfactant biosynthesis occurred mainly between 24hrs to 48hrs of suggesting that biosurfactant is produced as primary metabolites accompanying cellular biomass formation which accounted for the flunctuation in pH during degradation, similar to [56]. Ayanwu, [57] who reported that biosurfactant production is bacterial specific.High emulsification index and low surface tension facilitate the bioavailability of hydrophobic compounds to bacteria cells and thus enhance crude oil degradation [58,53]. The rate of biodegradation is dependent on the chemico-physical properties of the biosurfactants and not by the effects on microbial metabolism [59,60,61].Biometric flask experiments indicated that the CO2 production rates correlate with GC data, which is somewhat complex and time-consuming to produce thus the CO2 evolution rate provided an essential criterion for evaluating the optional methods for determination of crude oil biodegradation. Gas-Chromatography and mass spectrometry (GC-MS) elucidated degradation pattern of Bonny light crude oil. Before the degradation the main peaks were C1-C35 in Burkhoderia cepacia degraded n-C34-n-C35 within 7days at 37% in culture broth while Corynebacterium sp degraded n-C28-n-C35 and co-culture degraded n-C1 to n-C12 n-C22-n-C35 and n-C15 to n-C21 within the same day. Little or no reduction in total petroleum hydrocarbon was noticed in both isolate while n-C13 and n-C14 was degraded to n-C1 and n-C8 in their co-culture filtrate. Sathiskumar et al., [47] and Bello [62] reported that microbes preferably degrade and metabolise n-C8 to n-C15 n-akane followed by C16-C36 n-alkanes due to the simplicity of these hydrocarbon but this was contrary to this present finding, C34-C35 was noticed to degrade within 7days while other lower hydrocarbon were still present, this can be attributed to the autochthonous adaptation that allows microorganisms to be physically compatible with their habitat [63].This present finding revealed that Corynebacterium kutscheri has slight higher ability to degrade Bonny light crude oil than Burkhoderia cepacia such difference in the rate of hydrocarbon degradation may be due to presence of different catabolic genes involved in hydrocarbon degradation in the bacterial species [64,33].It is widely believed that individual organisms could only metabolize limited range of hydrocarbon substrates [65,66]. This has led to the assertion that mixed culture exhibited superior degradative potential than pure culture strains [38,66]. This often results in adaptation of the autochthonous organisms to the pollutants due to selective pressure and acquisition of degradative abilities [67] as reported by Salam et al., [39]Several reports [68,69,70,71,72] have confirmed microbial consortia as better degraders than pure isolates. In a culture, some species utilize intermediates of degradation of the original hydrocarbon produced by other members of the culture leading to a complete degradation of the oil [1,71]. Thus, a mixed culture is a better innoculum for bioremediation. The observation of diauxic growth phenomenon in the complex mixture of petroleum is expected as petroleum consists of linear as well as aromatic compounds which usually required different enzymes and biodegradation pathways. The utilization of the oil resulted in increase in both Optical density (OD6oo), protein content and dehydrogenase activities evidenced from the reduction in pH of the culture confirmed by chemical changes of the hydrocarbon substrates which must have caused by microbial enzymes [9,73,52]. Dehydrogenase was found to be synthesized in proportionate to growth and protein content.The enzyme activity is regulated through product inhibition, covalent modification and feedback inhibition [74]. Biodegradation of petroleum hydrocarbon generally relies upon the cooperation of more than a single bacterial species. This is particularly true when complete mineralization of the hydrocarbons to CO2 and H2O is desired. A pure bacterial culture may not have the metabolic capability to readily degrade certain compounds or to have the biomass necessary to degrade the compounds rapidly enough consortium of mixed populations with overall broad enzymatic capacities may be required to achieved total degradation of the petroleum.The higher biodegradation rate and microbial activity observed in the mixed culture may be related to shift in importance of one metabolic pathway over another possibly as a result of microbial synergism. The assumption that, more than one hydrocarbon compound degradation pathway exists in different microbial species and therefore, it is possible that individual bacteria able to degrade more than one aromatic substrate will have more than one pathway for their metabolism [75] (Shringfellow and Artken, 1995) This assertion is contrary to the present phenomenon. Williams, [76] reported that B.cepacia produced N-acetylhomoserine lactone an inducer that can stimulate quorum sensing mechanism in response to environmental challenges [77]. Since number of group of enzymes involved in complete mineralization of crude oil cannot be quantified, in this present finding the kind of synergism exhibited is neither protocooperation nor succession. GC- finger prints showed the same number of hydrocarbons was degraded throughout the experiment in their axenic culture while in their mixed culture degree of degradation was higher.However, the hypothesis that might explain this synergistical activities of these bacteria is quorum sensing mechanism such that the Corynebacterium kutscheri was able to intercept and transduce the quorum sensing signal produced by Burkholderia cepacia to control physiological interaction leading to gene expression that resulted into synthesis of various enzymes involved in mineralization of Bonny light crude oil. Both isolates produced surface active agents which might have very positively affected the uptake of substances by other hydrocarbons. For these reasons, mixed culture exhibited high degradation potential. There is no single strain of bacteria with the metabolic capacity to degrade all the components found within crude oil. In nature, biodegradation of a crude oil typically involves a succession of species within the consortia of microbes present. A combination of bacterial strain with broad enzymatic capabilities is required for active extensive degradation of crude oil. Oil degrading mixed cultures can be constructed by combining a number of strains with known degradative capabilities [79, 78], from the same ecological niche such that all the interaction that exist between them in their ecosystem can be maintained to enhance mineralization.
5. Conclusions
- These findings indicate that each bacterial strain possesses lower ability to utilize particular compounds but in their mixed culture, all the hydrocarbons were degraded within 10 days of incubation. The kind of interactions that influenced their degradative potential has been attributed to quorum sensing mechanism. Such combination can be formulated for the construction of bacterial consortia useful in bioremediation of refinery effluent
ACKNOWLEDGEMENTS
- The authors wish to thank the Board of Research of Ahmadu Bello University, Zaria, Nigeria for their financial support and Engr Alfred, U Amadi of Engineering & Technical Services Department, NNPC/KRPC Ltd for his technical support.
References
| [1] | Atlas, R.M. (1981). “Microbial degradation of petroleum hydrocarbons: environmental perspective”. Microbiological Reviews, 45: 180-209. |
| [2] | Amund, O.O., Adebowale, A.A. and Ugoji, E.O. (1987). Occurrence and characteristics of hydrocarbon utilizing bacteria in Nigerian soils contaminated with spent motor oil. Indian J. Microbiol., 27: 63-87. |
| [3] | Ward, D.M., Atlas, R.M., Boehm, P.D. and Calder, J.A. (1980). Microbial Biodegradation and The Chemical Evolution of Amoco Cadiz Oil Pollutants, Ambio, 9, 277-283. |
| [4] | Teal, J.M., Farrington, J.W., Burns, K.A., Stegeman, J.J. Tripp, B.W. Woodin, B. and Phinnley, C. (1992). The west Falmouth oil spill after 20 years; fate of fuel compounds and effects on animals. Mari. Poll. Bull., 24(12): 607-614. |
| [5] | Phillips, G.J.M., Stewart, J.E. (1974). Effect of four dispersants on biodegradation and Growth of bacteria on crude oil. Appl Microbiol., 284 (40):547-552. |
| [6] | Okpokwasili, G.C. and Okorie, B.B.(1988). Biodeterioration potentials of microorganisms isolated from engine lubricating oil.Tribol.inter.,21:215-217. |
| [7] | Ijah, U.J.J and Ukpe, L.I. (1992). Biodegradation of crude Oil by Bacillus strain 28A and 61B isolated from oil spilled soil. Waste management, 12(1): 55-60. |
| [8] | Ekpo M.A and Ekpo, E.I. (2006). Utilisation of Bonny light and Bonny medium crude oils by microorganisms isolated from Qua Iboe River Estuary. Nig. J. Microbiol., 20: 832-839. |
| [9] | Atlas, R.M. and Bartha, R. (1971).”Degradation and Mineralization of petroleum by two bacteria isolated from coastal waters. Biotechnology and Bioengineering,14(3): 297-308. |
| [10] | Okerentugba, P.O. and Ezeronye, O.U. (2003). Petroleum degrading potential of single and mixed microbial cultures isolated from rivers and refinery effluent in Nigeria Afr. J Biotechol., 2:288-292. |
| [11] | Ojumu, T.V, Bello, O.O., Sonibare, J.A. and Solomon, B.O. (2005). Evaluation of Microbial System for Bioremediation of petroleum refinery effluent in Nigeria Afr. J.Biotecnol., 4(1):31-35. |
| [12] | Adenipekun, C.O. and Fasidi, I.O. (2005). Bioremediation of oil-polluted soil by Lentinus Subnudus, a Nigerian white-rot fungus. Afr. J. Biotechnol., 4 (8): 796-798. |
| [13] | Ojo, O.A. (2006). Petroleum-Hydrocarbon utilization by native bacteria population from a wastewater canal southwest Nigeria. Afr J. Biotecnol., 5(4) 333-337. |
| [14] | Malik, Z.A and Ahmed, S. (2012). Degradation of petroleum hydrocarbons by oil field isolated bacterial consortium. African Journal of Biotechnology, 11(3):650-658. |
| [15] | Wagenet, R. J. and Bouma, J. (1996). The role of soil science in interdisciplinary research,SSSA Special Publication Number 45. |
| [16] | Capelli, S. M. Busalmen, P.J., and De Sanchez, R.S. (2001). Hydrocarbon bioremediation of a mineral based contaminated waste from crude oil extraction by indigenous bacteria. International bio-deterioration and biodegradation, 47:233-238. |
| [17] | Richard, J.Y. and Vogel, M.T. (1999). Characterization of a soil bacterial consortium capable of degrading diesel fuel. International biodeterioration and biodegradation, 44:93-100. |
| [18] | Kim, S. J., Choi, D. H. Sim, D.S and OH, Y. S. (2005). Evaluation of bioremediation effectivness on crude oil-contaminated sand. Chemosphere, 59: 845-852. |
| [19] | Borjana, K.T., George, R.I. and Nelly, E.C. (2001). Biosurfactant production by a new Pseudomonas putida strain. Z. Naturforsch, 57:356-360. |
| [20] | Kvenvolden, K.A., Rosenbauer, R.J. and Hostettler, F.D. and Lorenson, T.D (2000). Application of organic geochemistry to coastal tar residues from central California. Int. Geol. Rev., 42: 1-14. |
| [21] | Cameotra, S.S. and Pruthi, V. (1997). Production and properties of a biosurfactant synthesized by Arthrobacter protophormiae – an Antarctic strain. World J. Microbiol. Biotechnol.,13:137 139. |
| [22] | Atlas, R. M. (1995). Petroleum biodegradation and oil spill bioremediation. Mar. Poll. Bull., 31:178-182. |
| [23] | Sanni, G. O. and Ajisebutu, O. (2003). Biodegradation of Escravos light crude oil by some species of soil bacteria. Science focus,4:85-87. |
| [24] | Lee, K. and Levy, E.M. (1991). “Bioremediation: Waxy crude oils stranded on low-energy Shorelines.” Proceeding of 1991 International Oil Spill Conference .pp. 541-547. |
| [25] | Katarina, M. (2005). Isolation and Characterization of hydrocarbon degrading bacteria from Environmental habitats in western New York State. M.Sc thesis Rochester institute of Technology Rochester, NY 14623-5203. |
| [26] | Mandri, T. and Lin, T. (2007). Isolation and Characterization of engine oil degradig indigenous microorganism in Kwazulu- Natai South Africa. Afri J. Biotechnol., 61: 023-027. |
| [27] | Buchana R.E. and Gibbons, N.E. (1976) (Eds) Bergy’s manual of Determinative Bacteriology 8th Edition.Williams and Wilkins Co., Baltimore. |
| [28] | Ojo, O. A. (2006). Petroleum-Hydrocarbon utilization by native bacteria population from a waste water canal southwest Nigeria.Afr. J. Biotechnol.., 5(4):333-337. |
| [29] | Yakubu, M. B. (2007). Biodegradation of Lagoma crude oil using pig dung. African Journal of Biotechnology, 6 (24):2821-2825. |
| [30] | Bushnell, L.D. and Haas, H.F. (1941).The utilization of certain hydrocarbons by microorganisms Journal Bacteriol., 41:653-673. |
| [31] | Casida, L.E. (1977). Microbial metabolic activity in soil as measured by dehydrogenase determinations. Appl. Environ. Microbiol., 34:630–636. |
| [32] | Schnurer, J. and Rosswall, T. (1982). “Flurorescein Diacetate hydrolysis as a measure of total microbial activity in soil and litter”. Applied and environmental microbiology, 43(6):1256 – 1261. |
| [33] | Onuoha, S.C., Olugbue,V. U. Uraku, J.A. and Uchendu, D.O. (2011). Biodegradation Potentials of Hydrocarbon Degraders from Waste-lubricating Oil-spilled Soils in Ebonyi State, Nigeria. International journal of Agriculture and Biology, 13:586-590. |
| [34] | Cornfield, A. H. (1961). A simple technique for determining Mineralization of carbon during incubation of soil treated with organic material. Plant and Soil, 14:90-93. |
| [35] | Obire, O. and Nwaubeta, O. (2001). Biodegradation of refined petroleum hydrocarbons in soil .Journal of Applied Science &Environmental Management, 5 (1):43-46. |
| [36] | Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microorganism quantities of protein utilizing the principle of protein-dye binding. Anal biochem.,72:248 – 254. |
| [37] | Michaud, L., LoGiudice, A., Saitta, M. and DeDomenicovivia, M. (2004). The biodegradation efficiency on diesel oil by two psychrotrophic Antactic marine bacteria during a two- month-long experiment. Mar. Res. Bull., 49: 405-409. |
| [38] | Leahy, J. G. and Colwell, R. R. (1990). Microbial degradation of hydrocarbons in the environment. Microbiol. Rev., 54:305-315. |
| [39] | Salam, L. B., Obayori, O.S. Akashoro, O.S. and Okogie, G.O. (2011). Biodegradation of bonny light crude oil by bacteria isolated from contaminated soil. Int. J. Agric. Biol., 13: 245–250. |
| [40] | Sugiura, K.,Ishihara,M., Shimauchi,T. and Harayama, S. (1997) Physicochemical properties and biodegradability of crude oil. Environ. Scie. Technol., 31:45-51. |
| [41] | Okoh, A., Ajisebutu, S. Babalola, G. and Trejo-Hernandez, M.R. (2001). Potential of Burkholderia cepacia RQ1 in the biodegradation of heavy crude oil. Inter. Microbiol., 4:83-87. |
| [42] | Nnamchi, C. L., Obeta, J.A.N. and Eleogu, L.I. (2006). Isolation and characterization of some polycyclic aromatic hydrocarbon degrading bacteria from Nsukka soil in Nigeria. Int. J. Environ. Scie. Tech., 3(2):181-190. |
| [43] | Somtrakoon, K., Suanjit, S. Pokethitiyook, P. Kruatrachue, M. Lee, H. and Upatham, S. (2008). Enhanced biodegradation of anthracene in acidic soil by inoculated Burkholderia sp VUN10013. Curr. Microbiol., 57920:102-106. |
| [44] | Obayori, O.S., Adebusoye, S. A. Adewale, A.O. Oyetibo, G.O. Oluyemi, O.O. Amokun, R. A. and Ilori, M.O. (2009a). Differential degradation of crude oil (Bonny Light) by four Pseudomonas strains. J .Environ. Sci., 21:243-248. |
| [45] | Rahman, K.S. M., Raahman, J. T. Lakshmanaperumalsamy, P. and Banat, I. M. (2002). Towards efficient crude oil degradation by a mixed bacterial consortium. Bioresouce Technol., 85:257-261. |
| [46] | Thavasi, R., Jayalakshmi,S. and Banat, I. M. (2011). Effect of biosurfactant and fertilizer on biodegradation of crude oil by marine isolates of Bacillus megaterium, Corynebacterium kutscheri and Pseudomonas aeruginosa. Bioresources Technology, 102:772-778. |
| [47] | Sathishkumar, A., Arthur Raj, B. Sang Ho, B. and Sei-Eok, Y. (2008). Biodegradation of crude oil by individual bacterial strains and a mixed bacterial consortium isolated from hydrocarbon contaminated area. Clean, 36:92-96. |
| [48] | Bogan, B.W and Lamar, R.T. (1996). Polycyclic aromatic hydrocarbon-degrading capabilities of Phanerochaete laevis HHB-1625 and its extracellular ligninolytic enzymes. Appl. Environ. Microbiol., 62:1597-1603. |
| [49] | Wang, Q., Iriowen, E. Yuan, S. and Sparks, D. L. (2011). Impact of sediment from St Jones River, Delaware, U.S.A on microbial functional stability in two local soils. Bioremediation and Biodegradation, S:005. DOI:.org/ 10.4172/2155-6199. SI-005. |
| [50] | Bandick, A. K and Dick, R. P. (1999). Field management effects on enzyme activities. Soil biology and biochemistry, 31:1471-1479. |
| [51] | Lee, S.C., Lee, S.J., Kim, S.H., Park, I.H., Lee, Y.S., Chung, S. Y. and Choi, Y.L. (2008). Characterization of new biosurfactant produced by Klebsiella sp. Y6-1 isolated from waste soybean oil. Bioresour. Technol., 99(7): 2288-2292. |
| [52] | Sepahi, A.A., Dejban, G.I.. Emami, M. and Nakhoda, A.M. ( 2008). Isolation and characterization of crude oil degrading Bacillus spp. Iran J. Environ. Health Sci. Eng., 5:149-154. |
| [53] | Kumari, B., Singh, S.N. and Singh, D. P. (2012). Characterization of two biosurfactant producing strains in crude oil degradation. Process biochemistry, 47:2463-2471. |
| [54] | Nwanchukwu, S.U. and Ugodi, E. O. (1995). Impacts of Crude petroleum Spills on Microbial Communities of tropical soils. Intern J Ecol Eviron Sc.,21: 169-176. |
| [55] | Okpowasili, G.C.and James, W.A. (1995). Microbial Contamination of kerosene, gasoline,and Crude oil and their spoilage potentials. Material U organisamen, 29:147-156. |
| [56] | Abu-Ruwaida, A.S., Banat, I. M., Haditirto, S., Salem, A. and Kadir, M.S. (1991). Isolation of biosurfactant producing bacteria product, characterization and evaluation Biotechnological, 4:315-324. |
| [57] | Ayanwu, C.U. (2010). Surface activity of extracellular products of a Pseudomonas aeruginosa Isolated from petroleum contaminated soil. Inter. J. Environ. Science, 1(2):224-235. |
| [58] | Rosenberg, E. and Ron, E. Z. (1996). Bioremediation of petroleum contamination. In bioremediation, Principle and Applications, RL & D.L Crawford (eds) Cambridge University Press pp 100, 125. |
| [59] | Franzetti, A., Bestetti, G. Caredda, P. La Colla, P. and Tamburini,E. (2008). Surface-active compounds and their role in bacterial access to hydrocarbon in Gordonia strains. FEMS Microbiol. Ecology, 63:238-248. |
| [60] | Franzetti, A.,Caredda, P.,Rugggeri,C.,La Colla, P.,Tamburini, E.,Papacchini,M. and Bestetti,G. (2009). Potential application of surface-active compounds by Gordonia sp strain BS 29 in soil remediation technologies chemosphere. DOI:10.1016/J/ Chemosphere. 2012.12.052. (Article accepted in press). |
| [61] | Saliu, A., Abdulkadri, I. and Almustapha, M. N. (2009). An Investigation for potential development on biosurfactant. Biotechnology and Molecular Biology reviews, 3(5): 111-117. |
| [62] | Bello, Y.M. (2007). Biodegradation of Lagoma crude oil using pig dung. Afr. J. Biotechnol., 6:2821-2825. |
| [63] | Atlas, R. M. and Bartha, R. (1998). Fundamentals and Applications. In: Microbial Ecology.4th edition. Benjamin/ Cummings Publishing Company, Inc. California, USA, pp. 523- 530. |
| [64] | Majid, Z., Mnouchehr, V. and Sussan, K.A. (2008). Naphthalene metabolism in Norcadia otitidis caviarum strain TSH1a, a moderately thermophilic microorganisms. Chemosphere, 72:905-909. |
| [65] | Britton, L.N. (1984). Microbial degradation of aliphatic hydrocarbons. In: Gibson, D.T. (ed.), Microbial Degradation of Organic Compounds, Marcel Dekker Inc., New York. pp: 89–129. |
| [66] | Adebusoye, S.A., Ilori,M.O., O.O. Amund,O.O. Teniola, O. D and Olatope, S.O. (2007). Microbial degradation of petroleum in a polluted tropical stream. World J . Microbiol Biotechnol., 23: 1149–1159. |
| [67] | Wackett, L.P and Hershberger, L. C. D. (2001). Biocatalysis and Biodegradation, Microbial transformation of organic compounds, ASM press Washington, pp 228. |
| [68] | Obire, O. (1988). Studies on the biodegradation potential of some microorganisms isolated from water systems of two petroleum-producing areas in Nigeria. Nig. J. BOT., 1:81-90. |
| [69] | Amund, O.O. and Nwakaye, N. (1993). Hydrocarbon degradation potentials of yeast isolates from a polluted lagoon. J. Sci. Res. Dev., 1: 61-64. |
| [70] | Amund, O.O., Omole, C.A., Esiobu, N. and Ugoji, E.O. (1993). Effects of waste engine oil spillage on soil physico-chemical and microbiological properties. J. Sci. Res. Dev., 1(1): 61-64. |
| [71] | Facundo, J.M.R., Vaness, H. R. and Teresa, M. L.(2001). Biodegradation of diesel oil in soil by a microbial consortium. Water Air Pollution, 129:313-320. |
| [72] | Kulwadee, T., Vithaya, M., Prayad, P.and Attawut, I. (2001). Isolation and characterisation of crude oil degrading bacteria in Thailand. International Conference on ‘New Horizons in Biotechnology’, April 18-21,Trivandruim, India. |
| [73] | Oboh, B.O., Ilori, M.O.Akinyemi, J.O. and Adebusoye, S. A. (2006). Hydrocarbon degrading potentials of bacteria isolated from a Nigerian bitumen (Tarsand) deposit. Nature Sci., 4:51-57. |
| [74] | Brock, T. D and Madigan, M. T. (1991). Biology of micro-organisms, 6th ed. Prentice – Hall, New York, pp 171 – 175. |
| [75] | Stringfellow, W.T and Aitken, M.D. (1995). “Competitive metabolism of naphthalene,methy naphthalenes and Fluorene by phananthrene-degrading Pseudomonads” Applied and Environmental Microbiology, 6 (1):357-362. |
| [76] | Williams, P. (2007a). Bacillus subtilis: a shocking message from a probioticc. Cell Host Microbe, 1:248-249. |
| [77] | Atkinson,S. and Williams, P. (2009). Quorum sensing and social networking in the microbial world. Journal of Royal Society Interface, 6:959-978. |
| [78] | Foght, J.M., Semple, K, Gauthier, C. Wetlake, D.W.S. Blenkinsopp, S. Wang, Z. and Fingas, M. (1999). Effect of nitrogen source on biodegradation of crude oil by a defined bacterial consortium incubated under cold, marine conditions. Environ. Technol., 20: 839-849. |
| [79] | Komukai-Nakamura, S.K., Yamauchi, T.H. Inomata, Y.,Venkateswaran, K.T.H. Yamamoto,S. and Harayama, S. (1996). Construction of bacterial consortia that degrade Arabian light crude oil. J. Ferment. Bioeng., 82:570-574. |
| [80] | Kokub, D, Shafeeq, M., Khalid, Z.M.,Hussain A and Malik,K..A(1990).Comparative studies on emulsification and biodegradation on indigenous crude oils by enriched bacterial culture. Biorecovery, 2:55-68. |
| [81] | Margesin, R. and Schinner, F. (2001). Biodegradation and bioremediation of hydrocarbons in extreme environments. Appl Microbiol. Biotechnol., 56: 650- 663. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML