-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Microbiology Research
p-ISSN: 2166-5885 e-ISSN: 2166-5931
2014; 4(1): 24-27
doi:10.5923/j.microbiology.20140401.05
Production of Laccases by Fungi Isolated from Soil in Sokoto, Nigeria
Shinkafi S. A.1, Adamu U. S.1, Tanyi S. T.2
1Department of Microbiology, Usmanu Danfodiyo University Sokoto, Nigeria
2Department of Biological Sciences, Federal University Dutsin-Ma, Katsina, Nigeria
Correspondence to: Shinkafi S. A., Department of Microbiology, Usmanu Danfodiyo University Sokoto, Nigeria.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
A research was carried out on production of laccases by fungi isolated from soil within Sokoto, Nigeria. Soil samples were obtained from saw mill and were immediately transported to mycology laboratory for analysis. Carboxyl methyl cellulose (CMC) was applied on the media at different concentrations of 0.5g and 1g. on the media The fungi fungal isolates that can produce laccase were identified as Rhizopus oryzae, Fusarium sporotrichioides and Absidia corymbifera. From the results obtained, Rhizopus oryzae had the highest percentage frequency of occurrence of 50% followed by Fusarium sporotrichioides and Absidia corymbifera had with 12.5% each. Rhizopus oryzae and Fusarium sporotrichioides had a diameter of zone of inhibition of 43mm vertical and horizontal using both 0.5g and 1g carboxyl methyl cellulose (CMC). Absidia corymbifera had 17mm horizontal, 14mm vertical using 0.5g CMC and 18mm horizontal, 16mm vertical using 1g CMC. On the basis of the results found, it can be concluded that laccases can be produced by fungi isolated from the soil in our environment.
Keywords: Laccases, Fungi, Production, Soil
Cite this paper: Shinkafi S. A., Adamu U. S., Tanyi S. T., Production of Laccases by Fungi Isolated from Soil in Sokoto, Nigeria, Journal of Microbiology Research, Vol. 4 No. 1, 2014, pp. 24-27. doi: 10.5923/j.microbiology.20140401.05.
Article Outline
1. Introduction
- Laccases are multicopper enzymes belonging to the group of blue oxidases that use molecular oxygen to oxidize various aromatic and non-aromatic compounds by a radical-catalyzed reaction mechanism (Morozova et al., 2007). The enzymes are involved in the pathogenicity, immunity and morphogenesis of organisms and in the metabolic turnover of complex organic substances such as lignin or humic matter (Hattaka et al., 2001). Owing to their high non specific oxidation capacity, laccases are useful biocatalysts for diverse biotechnological application. Phylogenetically, laccases are members of the multi-copper protein family including ascorbate oxidase, ceruloplasmin and bilirubin oxidase. Because it belongs to the oxidase enzyme family, it requires oxygen as a second substrate for the enzymatic action. Laccase was first studied by Gabriel Bertrand in 1894.Laccases play a role in the formation of lignin by promoting the oxidative coupling of monolignols, a family of naturally occurring phenols. Others such as ones produced by the fungus Pleurotus ostreatus play a role in the degradation of lignin and can therefore be included in the broad category of ligninases (Condi, et al., 2007). They are widely distributed in higher plants, bacteria, fungi and insects. In plants, laccases are found in cabbages, turnip, potatoes, pears, apples and other vegetables.They have been isolated from Ascomyceteous, Deuteromyceteous and Basidiomyceteous fungi to which more than 60 fungal strains belong (Gianfreda et al., 1999).The white-rot Basidiomycetes fungi efficiently degrade lignin in comparison to Ascomycetes and Deuteromycetes which oxidize phenolic compounds to give phenoxy radicals and quinine (Eggert et al., 1996). In the recent years, these enzymes have gained application in the field of textile, food industry, paper and pulp industry, synthetic chemistry, cosmetics, biofuel cells, as a medical diagnostics tool, soil bioremediation and biodegradation of environmental phenolic pollutant and removal of endocrine disruptors (Couto et al., 2006). These enzymes are used for pulp delignification, pesticide or insecticide degradation, organic synthesis (Faccelo et al., 2008), waste detoxification, textile dye technological uses and biosensor and analytical application. In Nigeria, little or no effort has been made to improve the detoxification of environmental pollutants. This research work is therefore aimed at isolating and producing laccase enzyme from soil in our local environment to improve the detoxification of environmental pollutants.
2. Materials and Methods
2.1. Sample Collection
- A total of eight (8) samples of saw dust were collected from four different areas designated as; A3i, A3iii, B3iii, C3iii, C3ii, D3i, D3ii and D3iii. The samples were collected in clean polyethene bags and were immediately transported to Mycology laboratory of Usmanu Danfodiyo University Sokoto for analysis.
2.2. Serial Dilution
- One gram of each sample was diluted three (3) times in sterile physiological solution. Serial dilution was carried out in accordance with the procedure of Cheesbrough, (2000).
2.3. Isolation of Fungi
- The Suspension obtained after vigorous mixing of the of soil samples were left to stand for 30 mins. 0.5ml of different aqueous dilutions of soil suspensions were inoculated on Sabouraud Dextrose Agar plates. to which Streptomycin was had been incorporated to reduce bacterial contamination. The inoculated plates were incubated upright at 25°C for 5 days. All observed colonies were subculture to obtain and pure cultures were obtained.
2.4. Subculture
- The Colonies from the plates were observed for morphological difference. Each colony that differs morphologically from another was picked with a sterilized inoculating needle and inoculated on a freshly prepared Sabouraud Dextrose agar plates and incubated at 25°C for 3-5 days in order to obtain pure colonies of the isolates.
2.5. Identification of Fungi
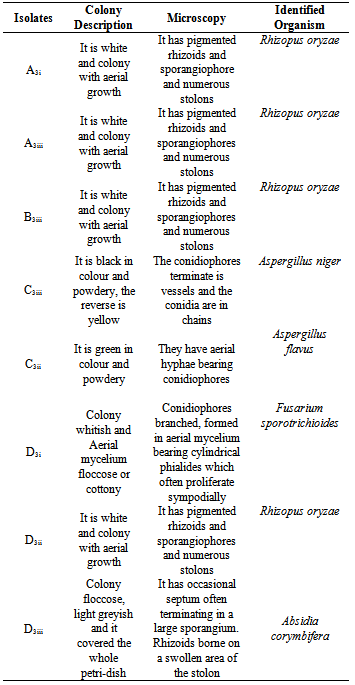
- The growth rate, colour, texture, colonial morphology and diffusible pigmentation of each sample were examined macroscopically. Tease mount using lactophenol cotton blue was adopted and microscopic features such as spore and hyphae morphology were observed and compared with the standard colored atlas as described by of Cheesbrough, (2000).
2.6. Production of Laccase
- A total of five (5) fungal strains were inoculated onto the plates containing plain agar with the following composition (g.l-1): peptone 3g, K2PO4 0.4g, MgSO4 0.5g, ZnSO4 0.001g, FeSO4 0.005g, MnSO4 0.5g and Carboxyl methyl cellulose (CMC) 0.5g and 1g respectively. The plates were incubated at 30°C for 5 days.
3. Results
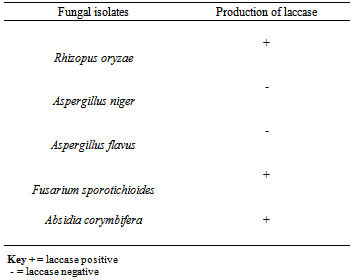
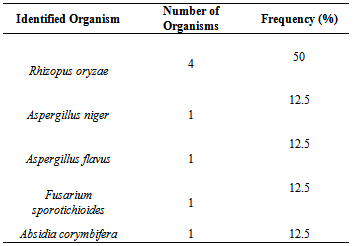
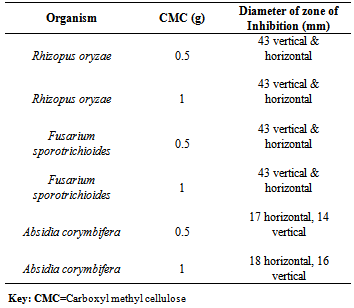
- A total number of five (5) species of fungi were isolated. Table 1 indicated the species of fungi isolated from the soil sampled. Table 2 indicated the results of fungal isolates producing laccase. The species include Aspergillus niger, Absidia corymbifera, Aspergillus flavus, Fusarium sporotrichioides and Rhizopus oryzae. 3 were laccase positive and 2 laccase negative. Table 3 showed the percentage frequency of occurrence of fungal isolates. From the results, it was observed that Rhizopus oryzae had the highest frequency of occurrence (50%) and Fusarium sporotrichioides, Aspergillus niger, Aspergillus flavus and Absidia corymbifera had 12.5% each.Table 4 showed the measurement of diameter of zone of inhibition of the organism. Carboxyl methyl cellulose (CMC) was used at different concentrations of 0.5g and 1g. From the result, Rhizopus oryzae and Fusarium sporotrichioides had 43mm vertical and horizontal using both 0.5g and 1g CMC. Absidia corymbifera had 17mm horizontal, 14mm vertical using 0.5g CMC and 18mm horizontal, 16mm vertical using 1g CMC.
|
|
|
|
4. Discussion
- A total number of five (5) species of fungi were isolated. Three (3) of the organisms were laccase positive. The laccase producing fungi include Fusarium sporotrichioides, Absidia corymbifera and Rhizopus oryzae. Aspergillus niger and Aspergillus flavus were laccase negative. The presence of this organism is not surprising as their source could be from soil or sawdust in our environment. From the research conducted it was found out that Rhizopus oryzae has the highest frequency of 50%, Fusarium sporotrichioides, Aspergillus niger, Aspergillus flavus and Absidia corymbifera had 12.5% each. Rhizopus oryzae and Fusarium sporotrichioides had a diameter of zone of inhibition of 43mm vertical and horizontal using both 0.5g and 1g carboxyl methyl cellulose (CMC). Absidia corymbifera had 17mm horizontal, 14mm vertical using 0.5gCMC and 18mm horizontal, 16mm vertical using 1g CMC. The findings of the present study agree with the works of Telke et al (2009) and Cullen and kersten (1996) who isolated similar groups of fungi from soil sample. From these findings, Penicillium rubrum, Stearum ostrea and Theliophora terristus were laccase positive while Aspergillus niger and Aspergillus flavus were not able to produce laccase enzyme. Laccase production by fungi has previously been shown to depend markedly on the composition of the cultivation medium for example carbon source, nitrogen content and phenolic inducer compounds have been reported to have significant effect on laccase production (Niku-paavola et al., 1990, Rogalski et al., 1991and Schlosser et al., 1997).
5. Conclusions
- Laccases are widespread in nature, being produced by a wide variety of plants, fungi and bacteria. The functions of the enzyme differ from organism to organism and typify the diversity of laccase in nature. Laccases catalyse the oxidation of phenolic compounds whilst simultaneously reducing molecular oxygen to water. The present study showed that laccase producing fungi were obtained from the soil in our environment. Base on the results obtained, it can be concluded that laccases can be produced by fungi isolated from the soil in our environment.
ACKNOWLEDGEMENTS
- Special thanks and appreciations go to our employer, Usmanu Danfodiyo University Sokoto, Nigeria. Our sincers gratitude also goes to the laboratory technologist who assisted us with the lab work.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML