-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Microbiology Research
p-ISSN: 2166-5885 e-ISSN: 2166-5931
2014; 4(1): 18-23
doi:10.5923/j.microbiology.20140401.04
Sero-Epidemiological Study of Camel Brucellosis in Mehoni District, South Eastern Tigray, Ethiopia
Habtamu Tassew, Fisseha Kassahun
Department of Veterinary Medicine, College of Veterinary Medicine, Mekelle University, Mekelle, Ethiopia
Correspondence to: Habtamu Tassew, Department of Veterinary Medicine, College of Veterinary Medicine, Mekelle University, Mekelle, Ethiopia.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
A cross-sectional study was conducted from November 2012 - August 2013 to investigate the prevalence and risk factors of camel brucellosis in Mehoni District, Southeastern Tigray, Ethiopia. From the total of 450 sera (300 camels and 150 goats) collected, 26 animals were positive by Rose Bengal plate test (RBPT), and 11 of 19 camels and 5 of 7 goats were confirmed by complement fixation test (CFT). The overall seroprevalence of Brucella antibodies in camels and goats was 5.78% (26) and 3.56% (16) by RBPT and CFT, respectively. The logistic regression analysis showed highly significant association of positive antibody status with potential risk factors of age (P = 0.021,X2 = 9.689), history of abortion (P = 0.001,X2 = 129.964), and parity number (P = 0.006,X2 = 12.475), and moderate associations based on herd size (p = 0.089, X2 = 4.848) and for keeping camels in close contact with goats (P= 0.082, X2 = 3.0281). In contrast, seropositivity was not associated with sex (P=0.532, X2 = 0.389) or, species (P= 0.857, X2 = 0.032) or herd size (P= 0.089, X2 = 4.848). Questionnaire interviews indicated that most of the animal owners were not aware of the zoonotic nature of brucellosis and they drank raw milk and do not take precautions in handling aborted foetuses. Clearly, further studies need to be conducted on the risk of human brucellosis in this area, to educate herders on zoonotic disease and to devise measures for disease control.
Keywords: Brucella,CFT, Risk factors, RBPT
Cite this paper: Habtamu Tassew, Fisseha Kassahun, Sero-Epidemiological Study of Camel Brucellosis in Mehoni District, South Eastern Tigray, Ethiopia, Journal of Microbiology Research, Vol. 4 No. 1, 2014, pp. 18-23. doi: 10.5923/j.microbiology.20140401.04.
Article Outline
1. Introduction
- Camels (Camelus deromedarius) are vital domestic animal species that are best adapted to harsh environments and fluctuating nutritional conditions of arid and extreme arid zones. These animals are endowed with extra ordinary features that enable them to survive and perform in such hard conditions[1]. Dromedaries are versatile living assets that ensure food security even during the dry periods and also serve as means of transportation and draught power[2]. Africa hosts 80% of the world population of dromedary (16.5 million), of which 63% attributed to east Africa[3]. According to the animal population census[4], the camel population in Ethiopia is estimated to be 2.314 million. The major ethnic groups owning camels in Ethiopia are the Beja, Rashaida, Afar, Somali and Borana[5]. Camels are kept in the arid lowlands of Ethiopia which cover approximately 61-65% of the total area of the country and, are the homes to 12-13 % of the total human population[6]. In drought stricken areas, ruminants are inferior to camels because of their physiological dependence on large amounts of water for metabolism and cooling. However, camels can retain lactation and produce high quality of milk under drought condition which makes them admirably suited to human requirements even when they are dehydrated and when other milk producing animals perish[7]. In spite of its vital importance particularly to the marginalized communities in the dry zones of tropics and subtropics, studies about camel are very few. Published information on diseases reveals that camels may be either carrier or susceptible or suffering from a vast array of infectious and parasitic diseases[8]. Brucellosis is one of widespread infectious disease of camel that has considerable public health importance as camel milk is consumed in raw. Brucellosis was reported in camel from different countries of Africa and Asia[9].Previous serological surveys showed overall prevalence rates of 4.4%[10] and 4.2%[11] in different camel rearing areas of Ethiopia. However, available studies on camel brucellosis are scanty and do not provide detail epidemiological information of the disease in the particular study area. Therefore, the present study were undertaken with the objectives to determine the sero-prevalence of camel brucellosis in Mehoni district and identify potential risk factors associated with the disease.
2. Materials and Methods
2.1. Description of the Study Area
- This study was carried out in Mehoni district, north eastern part of Ethiopia which is located in south eastern Tigray Regional State, near to border of Afar. Mehoni is situated approximately between 130151 and 130301N and 390301 and 390551E longitude, 200 km to south east of Mekelle, the capital of Tigray[12].
2.2. Study Population and Sample Size Determination
- Camel population in Mehoni district was represented the study population. However, the sample size required to determine the prevalence of camel brucellosis was determined by following standard formula recommended by Thrustfield[13]. N=1.962 Pexp(1-Pexp)/D2With 5% desired precision, at 95% confidence level and with expected prevalence of 50%, a total of 384 serum samples was supposed to be collected proportionally from three selected pastoral associations of the study district (Genete, Kukuftu and Chercher), however to increase the precision, the sample size has been increased to be 450.
2.3. Sampling Procedure
- The study district was selected purposively based on easiness for accessibility and camel population. Then, multi-stage sampling procedure was followed at three different stages to collect serum samples. The first stage is a primary sampling unit which represents each peasant association and was selected purposively based on the presence of camel population and easiness for accessibility. In the second and third stages; following proportionalization, camel herds and individual camels was selected randomly from each peasant association and herd, respectively.
2.4. Data Collection
2.4.1. Questionnaire Survey
- One hundred randomly selected camel owners from three pastoral associations of Mehoni district was interviewed by using structured questionnaire. Two questionnaire formats; one for serum sampled individual animal history and the other structured questionnaire format for herders were developed. By doing so; risk factors that have possible association with the occurrence of brucellosis were investigated and used to support serological results.
2.4.2. Serological Survey
- About 10 ml of blood was collected from the jugular vein using plain vacutainer tubes. While collecting the sample (specific animal identification, sex, age, etc) was labeled on the tube and the tubes was left overnight to clot at room temperature and finally the serum was carefully separated by decanting on cryovials and stored in a refrigerator at -20℃ until the time of testing. Rose Bengal Plate Test (RBPT) All sera samples collected was initially screened by RBPT using RBPT antigen (Institut Pourquier 325, rue de la galèra 34097 Montpellier cedex 5, France) by following the standard procedure recommended by Nielson and Dunkan [14]. Sera samples were kept in a refrigerator at +4℃ before testing. Sera and antigen were left at room temperature for half an hour before the test to maintain to room temperature. Complement Fixation Test (CFT) Those positive sera with RBPT were further tested with CFT for confirmation using Standard Brucella abortus antigen (CVL, New Haw, Weybridge, Surrey KT15 3NB, UK). The CFT test proper and reagent preparation procedures were done by following the procedures outlined by OIE[15]. The reading was as complete fixation (no hemolysis) with water clear supernatant was recorded as + + + +, nearly complete fixation (75% clearing) as ++ +, partial hemolysis (50%) + + and some fixation (25% clearing) as +. Complete lack of fixation (complete hemolysis) was recorded as 0. For positive reactions final titration was recorded.
2.5. Data Management and Analysis
- Different models or analytical tools were employed to analyze collected data on STATA version 16 Software. Descriptive statistics were used to analyze majority of the data collected by questionnaire. Chi-square test was used to rule out whether there was significant association between prevalence of camel brucellosis and different groups of sex and herding experience. In addition, General linear Model (GLM) procedure with t-test and Duncan’s multiple range tests were used to test differences of disease prevalence amongst different age, parity and herd size groups.
3. Results
3.1. Overall Seroprevalence
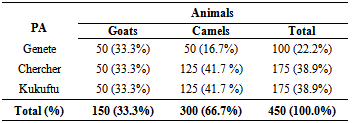
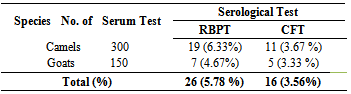
- In this study, 450 sera (300 camels and 150 goats) were collected from three peasant associations (Genete, Chercher and Kukuftu) (Table 1). Using RBPT, 26 animals (5.8%) were identified as seropositive reactors from the total serum sample collected. The seropositive reactors with RBPT were subjected for further CFT confirmation. Accordingly, 16 (3.56%) overall seropositive reactors were detected by CFT (Table 2).
|
|
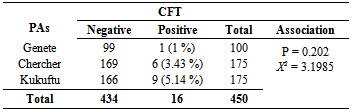
3.2. Risk Factors and Seroprevalence
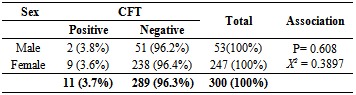
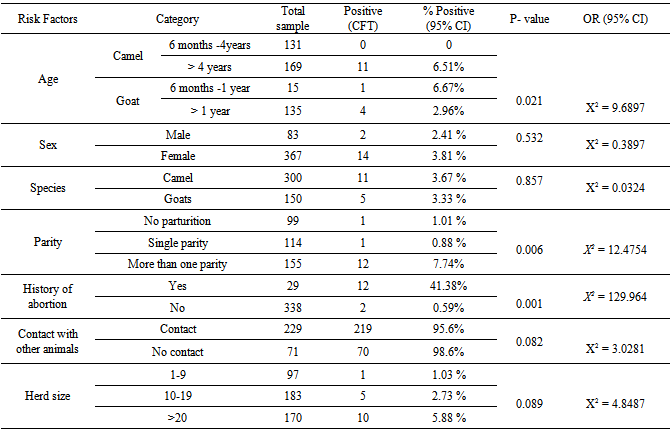
- To identify the potential risk factors association with the occurrence of camel and goat brucellosis, all breeding male and female camels and goats above six month of age were included. From the total camels tested, 247 (82%) were female and 53 (17.7%) were male camels. The seroprevalence of brucellosis in male camels is 3.8%, a slightly higher than female (3.6%), however; there was no statistically significant difference observed (P= 0.608, X2 = 0.3897) (Table 3).
|
|
|
3.3. Questionnaire Interviews
- About 100 owners of animals have been interviewed regarding the potential risk factors and their awareness about the public health impact of the disease during blood sample collection and history recording. This study showed that camels were commonly reared with small ruminants especially of goats and this might increase the spread of the disease among animals. Most of the respondents did not have any awareness about the zoonotic nature of the disease and they drank raw milk and did not take care of handling aborted foetus. There was no data of vaccination against camel and goat brucellosis in the study area.
4. Discussion
- Brucellosis is a widespread zoonotic disease that still of veterinarian, public health and economic concern in many developing countries including Ethiopia[16, 17, 18]. Brucellosis is a classical zoonosis and the major sources of infection remain contact with infected animals or handling of carcasses and less frequently through food. Camels are not known to be primary hosts of Brucella organisms but they are susceptible to both B. abortus and B. melitensis[19]. The seroprevalence of brucellosis in camels appears to follow two distinct patterns: a low (2-5%) prevalence in nomadic or extensively kept camels and a high (8-15%) prevalence in camels kept intensively or semi-intensively[9]. In this study, 3.67% seroprevalence of camel brucellosis was observed which is in close agreement with Bekele[20], Teshome et al.[11] and[10] who reported prevalence rates of 0.4-2.5%, 4.2% and 4.4%, respectively in Borena, Oromia region and with Ghanem et al.[21] who reported a prevalence of 3.1% in Somalia. As most of camels are kept by nomadic people despite the variation in region or locality where all areas practice extensive farming system which agrees with the report of Abbas and Agab[9] that seroprevalence was low in this study. In contrary to the present study, there was relatively high seroprevalence (5.5%) by Richard[22] in Afar region and in other camel-rearing areas of Ethiopia and (7.6%) by Sisay et al.[23] in different districts of Afar region. Brucellosis in camels has been reported in many countries with different seroprevalences: in Kenya, a prevalence rates of 4.6-10.3% by Kagunya and Waiyaki[24]; in Sudan, a prevalence of 8.0% by Osman and Adlam[25]; in Egypt, 10-20% and Saudi Arabia, 4.3-8.6% by Radwan et al.[26]. These varying reactor rates for camel brucellosis in different countries might be due to varying in husbandry and management practices, susceptibility of the animal, virulence of the organism, presence of the reactor animals in the region, absence of veterinary service, lack of awareness by the nomads about the disease and the pastoralists’ movement from place to place. The movement of animals may worsen the epizootic situation of brucellosis in an area as the movement contributes on disease spread from one herd to another due to the movement of an infected camel in to a susceptible camel herd[27]. Seroprevalence of brucellosis in relation to sex of animals as some of the researchers reported significantly higher prevalence in females than in males[28] while others in Sudan[29] and Saudi Arabian[26] reported that male camels have high antibodies against Brucella infection more frequently than females. In this study, even though the logistic-regression analysis indicated that there was no statistical significant difference between the two groups, males showed relatively higher prevalence (3.8%) than female groups (3.6%) which is in agreement with the later findings.Infection may occur in animals of all age groups but persists commonly in sexually matured animals. Younger animals tend to be more resistant to infection and frequently clear infection although few latent infections may occur[27]. The present study showed that there was slightly higher significant association with the occurrence of the disease in adult (> 4 years) than young camels (6 month to 4 years). The low seroprevalence in young camels might be because of maternal immunity. Susceptibility appears to be more commonly associated with sexual maturity and risk of infection increases with pregnancy as the stage of pregnancy increases[30]. A higher seroprevalence (4.4%) was observed in camels reared with small ruminants (goats) as compared to those kept with no contact with small ruminants (1.4%) and there was statistically moderate significant association between camel groups with small ruminants and without ruminants (P=0.082, X2 = 3.0281). A significant association has been reported by Andreani et al.[31] in Somalia where high chance of Brucella transmission from small ruminants to camels since they were in free range proximity in the bush and watering points. A contributing factor to the spread of the disease may be the movement of animals for grazing and watering during the dry season as aggregating animals around watering point will increase the contact between infected and healthy animals and thereby facilitate the spread of the disease[22].The classical symptoms of brucellosis in camels are abortion, placental retention, still birh, delayed sexual maturity and infertility[32]. In the present study, the seroprevalence in aborted camels and goats was 41.38% which is in close agreement with the findings of Mohammed[16] where he reported seroprevalence of 40% in camels with abortion in and around Dire Dawa city, Eastern Ethiopia.There was statistically significant association (P = 0.006, χ2 = 12.4754) between parity and the seroprevalence of the disease. Those she-camels and goats with the history of more than one parity were 1.59 times more at risk of being seropositive to Brucella infection than those with no parturition (OR = 1.594; 95% CI, 0.944 – 2.694). Those she-camels and goats with single parity were 1.25 times more at risk of being seropositive than those with no history of parturition. Higher infection rate was recorded in the she-camels and goats which gave birth to more than one calf (7. 74%) than those with single parity (0.88%) and with no parity (1.01%). The present study is therefore, in consistent with the previous study by Bekele[20] where higher reactor rate was recorded in camels with more than one parity number, compared to other group of camels. In conclusion, brucellosis is an important re-emerging bacterial zoonosis and a significant cause of reproductive losses in animals, and camel brucellosis is one of a widespread disease in camel rearing areas of Ethiopia. The present study provided a baseline data or status of camel brucellosis in Mehoni District and showed the potential risk factors that would contribute to the occurrence of the disease in camels as well as possible zoonotic implications in human beings. The overall seroprevalence was relatively low as compared to many other research findings. In this study, different age groups, parity number and history of abortion showed statistically high significant association with the prevalence of the disease; however, the association with different peasant associations, sex and species of the animal was not statistically significant with the occurrence of the disease except a slight significant difference with herd size and in camels co-exist with small ruminants. Lack of awareness about the zoonotic nature of brucellosis together with existing habit of raw milk consumption and, close contact with animals can serve as means of infection to human beings. In view of the above facts, the following points should be considered in controlling of the disease:• Camel pastoralists are often neglected from public services, facilities and information. Thus, awareness about modern animal husbandry, disease prevention and risk of zoonotic diseases is quite necessary.• Further researches that intended for the isolation of causative agents and identification of species and biotypes in Ethiopia are important.• Camels prosper, produce and sustain the life of the pastoralists under a number of constraints. Hence, researches that support these animals and maximize their performance are recommended.• Adequate brucellosis control programs in small ruminants would contribute to the reduction of the disease prevalence in camels.
ACKNOWLEDGEMENTS
- This project was funded by the US Agency for International Development (USAID) project, the Livestock- Climate Change Collaborative Research Support Program (LCC CRSP) in Sub-Saharan Africa and South Asia in collaboration with Colorado State University. The authors would like to thank to the US agency (USAID) and Colorado State University for funding this project. Grateful thanks to Prof. Richard, Director of the program (LCC CRSP) for mentoring the project throughout the study and Dr Solomon Desta and Dr Hoag Dana for their unreserved support. It would be grateful to thank Mekelle University for providing all the necessary facilities. Many thanks are to Mehoni District veterinarians and Dr Yohannes Hagos for all their contribution during the study period.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML