-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Microbiology Research
p-ISSN: 2166-5885 e-ISSN: 2166-5931
2014; 4(1): 14-17
doi:10.5923/j.microbiology.20140401.03
Original Anti-Microbial Treatment of Purulent-Inflammatory Lung Diseases in Patients Supported by Long-Term Artificial Ventilation of Lungs
F. G. Nazirov, R. A. Ibadov, Z. A. Shanieva, A. Sh. Arifjanov, A. D. Eshonhodjaev, T. B. Ugarova, N. A. Strijkov, P. G. Komirenko
Republican Specialized Center of Surgery named after ac.V.Vahidov, Tashkent, Uzbekistan
Correspondence to: Z. A. Shanieva, Republican Specialized Center of Surgery named after ac.V.Vahidov, Tashkent, Uzbekistan.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
We analysed the spectrum of microbial agents causing bronchial-pulmonary complications in 58 patients who underwent various surgical interventions and were supported by long-term artificial ventilation of lungs. “FarGALS”, a medication with high antimicrobial activity developed and produced at V.Vakhidov Republican Specialised Centre of Surgery, was used for the first time in the nebuliser therapy. In comparison with other antimicrobial agents, the use of “FarGALS” has reduced complications of long-period artificial ventilation, justifying further investigations of this medication for the use in nebuliser therapy by intensive care units (ICU).
Keywords: Anti-microbial Treatment , Intensive Care , Nebuliser Therapy
Cite this paper: F. G. Nazirov, R. A. Ibadov, Z. A. Shanieva, A. Sh. Arifjanov, A. D. Eshonhodjaev, T. B. Ugarova, N. A. Strijkov, P. G. Komirenko, Original Anti-Microbial Treatment of Purulent-Inflammatory Lung Diseases in Patients Supported by Long-Term Artificial Ventilation of Lungs, Journal of Microbiology Research, Vol. 4 No. 1, 2014, pp. 14-17. doi: 10.5923/j.microbiology.20140401.03.
Article Outline
1. Introduction
- With a duration of mechanical ventilation of lungs risk of pneumonia increases. Thus, according to an epidemiological study EPIC II (Russia), which included more than 14 thousand ICU patients have shown that every second patient have some signs of infection. In this case, 70% of patients the source of infection is the lungs.[2] Among the factors determining the etiological structure of bronchopulmonary complications, above all, should be made prior antimicrobial therapy and duration of mechanical ventilation[11]. Thus, according to Engelgart, only 20% of hospital-acquired pneumonia were of exogenous origin. An important role is played by translocation conditionally pathogenic bacteria in the gastrointestinal tract. At various critical conditions developed ischemia of the intestinal wall, broken motor and intestinal barrier function. In these conditions, the penetration of bacteria and their toxins in the portal and systemic circulation occurs. In addition, the observed retrograde colonization of the overlying parts of the intestinal tube. It is also submitted real that limfogematogen way of spreading bacteria from extrapulmonary sites of infection.It is conclusively proven, that early adequate empirical antibiotic therapy (EAT) for LAVL significantly reduces the risk of VAP and adverse outcome[3].The important and urgent is the problem of prevention of scar stenosis of the trachea occurs after prolonged mechanical ventilation. Etiopathogenic found that as a result of ischemia tracheal mucosa in the area of localization inflatable cuff endotracheal tube developed granulation and cicatricial stricture[2]. Violation of purulent sputum expectoration of the tracheobronchial tree in the presence of tracheal stenosis worsens during the process, in the area of tracheal mucosal injury occurs pathological focus accession nosocomial infection.The correct choice of empirical EAT scheme is crucial, but a variety of reasons of acute respiratory failure, the differences in the severity of the initial status of the patients, especially the earlier EAT most significantly affect the etiology of chronic inflammatory diseases of the lungs and makes it difficult, so the search for effective measures to prevent and treatment remains an urgent problem in modern resuscitation.
2. Objective
- The aim of this study was to evaluate the effectiveness of the proposed algorithms predict, prophylaxis and treatment of bronchopulmonary complications in prolonged mechanical ventilation.
3. Materials and Methods (Experimental)
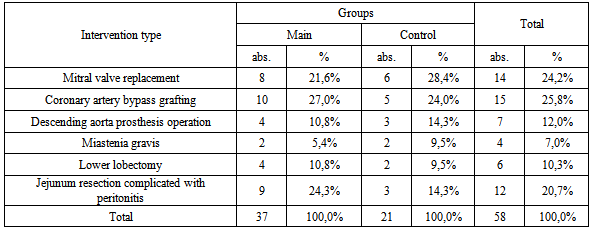
- The material for the study is based on the results of bacteriological studies of crops from the trachea, throat, bronchial washings 58 operated patients who were on PIVL for 2010-2011-2012 (I-IX months.) years in the RSCS named after ac. V.Vahidov. The patients were divided into two groups: the main group included patients who used domestic product "FarGALS" (in 1:4 dilution) as inhalation therapy and bronchial lavage passed through the instrument channel fiberoptic (37 patients) in the control group included Patients treated by traditional methods (nebulizer with antibacterial drugs, bronchodilators and mucolytic - 21 patients). The mean age was 37,3 ± 12,6 years. The study included patients after various operations, the nature of which is presented in Table 1.
|
4. Results and Discussion
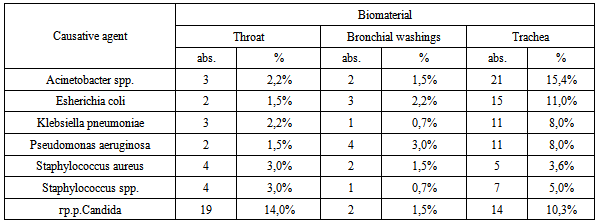
- Total examined 340 samples of clinical material, of which 136 (40.0%) samples were identified different types of microorganisms (Table 2).
|
|
5. Conclusions
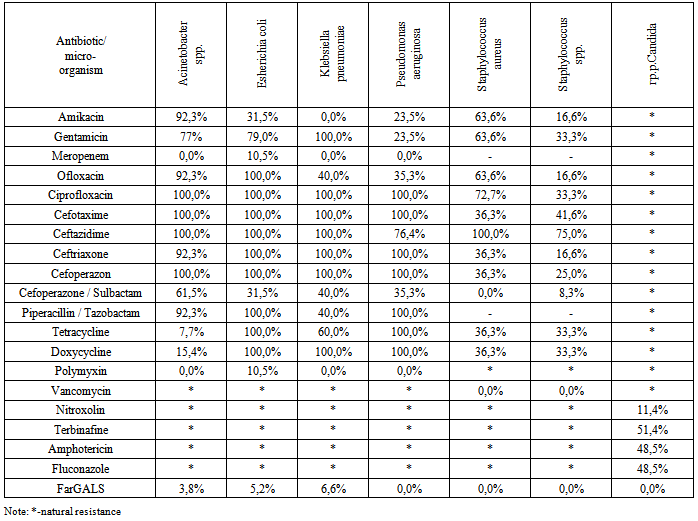
- In patients on prolonged mechanical ventilation, the tendency of the prevalence of gram-negative microorganisms planting, and for the last two years, the array of multi-resistant flora released. Gram-negative microorganisms isolated cultures are resistant to a broad range of antibiotics. Analysis of the antimicrobial activity of the domestic drug "FarGALS" against all tested crops (gram-positive organisms, gram-negative microorganisms, fungi), shows a high sensitivity.Inclusion in the complex post-operative prophylaxis and treatment of inflammatory lung disease in patients on prolonged mechanical ventilation drug "FarGALS" in the form of inhalation therapy and Fibro-broncho-kopic bronchial lavage reduces the incidence of early and late specific bronchopulmonary complications from 23% to 8%, achieve clinical improvement of the patients already at 2-3 days and shorten recovery period from 8-10 to 5-6 days. High activity of the domestic drug "FarGALS" against multiresistant strains of causes feasibility of further study and application of patients are on LAVL.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML