-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Microbiology Research
p-ISSN: 2166-5885 e-ISSN: 2166-5931
2014; 4(1): 6-13
doi:10.5923/j.microbiology.20140401.02
Antimicrobial Activities of Plant Extracts against Methicillin-Susceptible and Methicillin-Resistant Staphylococcus aureus
E. L. Chuah1, Z. A. Zakaria1, Z. Suhaili2, S. Abu Bakar1, M. N. M. Desa1, 3
1Department of Biomedical Science, Faculty of Medicine and Health Sciences, UniversityPutra Malaysia, Serdang, Selangor, Malaysia
2School of Animal Science, Faculty of Agriculture, Biotechnology and Food Science, UniversitySultan ZainalAbidin, Gong Badak Campus, Kuala Terengganu, Malaysia
3Halal Products Research Institute, UniversityPutra Malaysia, Serdang, Selangor, Malaysia
Correspondence to: M. N. M. Desa, Department of Biomedical Science, Faculty of Medicine and Health Sciences, UniversityPutra Malaysia, Serdang, Selangor, Malaysia.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
The present study was carried out to compare the antimicrobial activities of methanol leaf extracts of Bauhinia purpurea, Dicranopterislinearis, Melastomamalabathricum and Muntingiacalabura portrayed by different antimicrobial assays against methicillin-susceptible and methicillin- resistant Staphylococcus aureus strains. Antimicrobial activities of the methanol leaf extracts were preliminarily screened by disc diffusion. Minimum inhibition concentration (MIC) and minimum bactericidal concentration (MBC) were determined by broth microdilution and colorimetric assay (resazurin). Based on disc diffusion method, S. aureusATCC®700699™ (MRSA) elucidated higher susceptibility pattern against all plant extracts compared to S. aureusATCC®25923™ (MSSA). Taking results from all employed assays into consideration, M. calaburamethanol leaf extract comparably elicited the highest antimicrobial activity than the other methanol leaf extracts against both microorganisms. The MIC values were determined by colorimetric assay (resazurin) due to pigmentations of the methanol leaf extracts that obscured visual growth turbidity inspection. Complication in colour changes observation in colorimetric assay to determine MBC was overcome by employing the conventional plating method. This study suggested that all antimicrobial assays should be carried out concurrently so as the data obtained can be comparatively analysed for a better outcome as each antimicrobial assay has its own shortfall.
Keywords: Antimicrobial activities, Disc diffusion, Broth microdilution, Colorimetric assay, Bauhinia purpurea, Dicranopterislinearis, Melastomamalabathricum, Muntingiacalabura, Staphylococcus aureus
Cite this paper: E. L. Chuah, Z. A. Zakaria, Z. Suhaili, S. Abu Bakar, M. N. M. Desa, Antimicrobial Activities of Plant Extracts against Methicillin-Susceptible and Methicillin-Resistant Staphylococcus aureus, Journal of Microbiology Research, Vol. 4 No. 1, 2014, pp. 6-13. doi: 10.5923/j.microbiology.20140401.02.
Article Outline
1. Introduction
- Bacterial and fungal pathogens are the etiological agents of human infections[1] which have raised concern in the healthcare field over the years, particularly those which have adopted resistance towards antimicrobial agents. The increasing antimicrobial resistance is due to genetic mutations, or acquisition of antibiotic resistance determinantsand stimulated by the misuse of antimicrobials in drug prescriptionsand for other purposes[2]. Resistant isolates weredated back in the late 1950s and early 1960s following the initiation of the use of β-lactamase-stable cephalosporins and semi-synthetic penicillins in treating diseases caused by S. aureus[3], narrowing down the treatment options. One notable example is the methicillin- resistant S.aureus (MRSA) which frequently displayed multidrug-resistance properties. The acceptance and practices of traditional medications in many countries[4] have burgeoned the interest to look for alternatives from plant sources to overcome the matter[5, 6]. Such measure could possibly lead to new findings to treat diseases in lieu of depending solely on drug developments which may be time-consuming aside from causing side effects to human health after prolonged treatments. Malaysia is the home of wide diversity of flora due to its constantly warm and humid equatorial climate throughout the year, housing various plants with medicinal properties. Plants such as Bauhinia purpurea, Dicranopterislinearis, Melastomamalabathricum and Muntingiacalabura are no strangers in household remedies since the ancestry era. Bauhinia purpurea is a flowering plant belonging to the family Leguminosae which is commonly found in Southeast Asia. Traditional medical practitioners have been using it to treatan army of diseases such as skin diseases, diarrhoea, ulcers and rheumatism[1, 7, 8]. Dicranopterislinearis is one of the members in the fern family Gleicheniaceae. It has been used extensively in the traditional medication practices in South East Asia to reduce fever and to treat broils, wounds, ulcers, intestinal worms infection as well as asthma[9]. Melastomamalabathricumis a flowering plant from the family Melastomataceae. It has been used traditionally to treat piles, diabetes, diarrhea and wounds, lowering blood pressure and prevent smallpox scars[10, 11]. Muntingiacalabura falls in the flowering plant family Elaeocarpaceae. It is traditionally used in Cambodia and Malaysia to promote menstrual discharge and abortion. It is also used in treatment of liver diseases, headache and cold as well as acting as antidyspeptic, antispasmodic, antihysteric, tranquilizer and diaphoretic[12, 13]. Previous studies have found that these plant elicited many bioactivities, namely antioxidative[7, 10, 14, 15], cytotoxicity[12, 16-18], anti-inflammatory, antinociceptive and antipyretic[19-23]. Many parts of these plants, typically the leaf, flowers and roots, have been previously studied for their bioactivities contributing to the field of pharmacology, namely antimicrobials[7, 10, 14, 16]. Nevertheless, many previous studies usually accessed individual plant and a single method to study on the antimicrobial activity. Considering the wide range of plants with potential antimicrobial activities, it is of interest to identify the efficacy of the plants in comparison to one another and the outcome when assayed by different methods. Thus, this study broadens the spectrum by comparing four different plants (B. purpurea, D. linearis, M. malabathricum and M. calabura) to study on their antimicrobial activities using three experimental models (disc diffusion method, broth microdilution method and the employment of resazurin). Previous studies have also shown that methanol plant extracts frequently exhibited the highest antibacterial activity[1, 24]. Therefore this study preliminarily attempts to evaluate the antimicrobial activities of the four plants by focusing on their methanol leaf extracts against methicillin-susceptible S.aureus (MSSA) and MRSA.
2. Materials and Methods
2.1. Plant Materials
- Fresh leaves of B. purpurea, D. linearis, M. malabathricum and M. calabura were collected from their natural habitats around University Putra Malaysia area. Plant samples were sent for verification and voucher specimens SK 2028/12, SK 2029/12, SK 2046/12 and SK 2047/12 were deposited at the Biodiversity Unit of Institute of Biosciences, UniversityPutra Malaysia for verification purposes.
2.2. Methanolextraction of Plants
- The plant samples were washed under tap water, rinsed with distilled water and dried in drying oven at 40°C. Upon grinding into powder form, each plant sample was weighed and soaked in absolute methanol at the ratio of 1:20 (w/v) for 72 hours. Extracts were then filtered through Whatman No. 1 filter paper to separate the plant residues, followed by rotary evaporation to remove methanol from the crude methanol leaf extracts. The dried methanol leaf extracts were stored at 4°C until further use for antimicrobial activities screening.
2.3. Bacterial Strains
- The test bacteria employed in this study were from American Type Culture Collection (ATCC®); S. aureusATCC®25923™ (MSSA) and S.aureusATCC®700699™ (MRSA). Both bacterial strains were maintained in viable state via inoculation on Mueller-Hinton Agar (MHA) and overnight incubation at 37°C.
2.4. Preparation of Inoculums
- A loopful of bacterial colonies from overnight solid media culture was inoculated into sterile Mueller-Hinton Broth (MHB). Broth inoculums were incubated at 37°C for 16-20 hours prior to antimicrobial testing.
2.5. Preliminary Antimicrobial Screening
2.5.1. Disc Diffusion
- Broth inoculums were standardized to 0.5 McFarland standard by means of dilution with sterile MHB and measuring the optical density measurement at 625 nm wavelength. Absorbance readings were fixed to be within the range of 0.08 - 0.13 (equivalent to approximately 1.5 x 108 CFU/mL). Standardized broth inoculums were lawned onto sterile MHA by swabbing with sterile cotton swabs. Six mm sterile paper discs were impregnated with methanol leaf extracts (pre-dissolved in 10% dimethyl sulphoxide (DMSO)) to achieve final concentrations of 1 mg, 5 mg, 10 mg, 15 mg and 20 mg per disc.Six mm sterile paper discs loaded with 10% DMSO were used as growth control. The discs were placed aseptically and distinctively onto the inoculated MHA plates. Agar plates were incubated at 37 °C for 16-18 hours and inhibition zones formed were measured and compared with those of commercial antibiotics (gentamicin and tetracycline). Test was performed in triplicates. Methanol leaf extracts which showed presence of antimicrobial activities against the bacterial strains tested were subjected to broth microdilution and colorimetric assay to evaluate their respective minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) values.
2.6. Determination of MIC and MBC Values
2.6.1. Broth Microdilution
- Each well of 96-well microtiter plate was aliquoted with 50 μL of Mueller-Hinton broth (MHB); 12th well (sterility control) was added with 100 μL of MHB. 11th well (growth control) was added with MHB with 10% DMSO. 50 μL of methanol leaf extract initially dissolved in 10% DMSO to the concentration of 40 mg/mL was added into the first well and a serial 2-fold dilution was performed by transferring 50 μL of the suspension to the subsequent wells up till the 10th well; the final 50 μL of the suspension was discarded. Broth inoculum was standardized according to the protocol by Wiegand et al.[25]. 0.5 McFarland standard broth inoculum was diluted to the ratio of 1:100 and added into 1st-11th well to achieve the final inoculum size at 5 x 105 CFU ml-1. Methanol leaf extracts were tested at the highest concentration of 10 mg/mL and the lowest being 0.020 mg/mL against test organisms. Absorbance reading at 625 nm wavelength for each plate was measured pre- and post-incubation at 37°C for 16-18 hours. Bacterial cell viability and minimum inhibitory concentration (MIC) values were determined by observing the turbidity and the absorbance reading of the suspension post-incubation. The lowest concentrations of methanol leaf extracts with clear suspensions were considered as the MIC values, whereas the suspensions treated with methanol leaf extracts which showed no increment of absorbance readings post-incubation time were considered as the MIC values. The lowest concentrations of methanol leaf extracts in post-incubation suspensions which did not harbour any bacterial growth upon spotting on MHA plates (conventional plating method) after overnight incubation at 37 °C were considered as the minimum bactericidal concentration (MBC) values. Test was performed in triplicates alongside commercial antibiotics (gentamicin and tetracycline) as positive controls.
2.6.2. Colorimetric Assay
- Following incubation in broth microdilution method, 30 µL of 0.01% resazurin was added into each well of the 96-well microtiter plates and the plates were subjected to incubation for another two hours. Colour change of the dye from blue to pink indicated cell viability. The suspensions treated with lowest methanol leaf extract concentrations which changed the resazurin colour from blue to pink or purple were considered as the MIC values whereas the ones treated with the lowest methanol leaf extract concentrations which did not change the blue pigmentation of resazurin was considered as the MBC values. MIC and MBC values obtained via colorimetric assay were compared to those obtained from the visual turbidity observation and conventional plating method respectively in broth microdilution method. Test was performed in triplicates and concurrently with commercial antibiotics (gentamicin and tetracycline) as positive controls.
2.7. Statistical Analysis
- Measurements of inhibition zones and absorbance readings obtained from the disc diffusion method and broth microdilution method were analysed individually using the Scientific Package of Social Science (SPSS) software. All triplicate data were presented in mean ± Standard Deviation (S.D) manner. Whenever possible, Mann-Whitney and Kruskal-Wallis tests were employed for comparing two or more than two variables groups respectively. The level of significant difference between mean values was set at p-value < 0.050.
3. Results
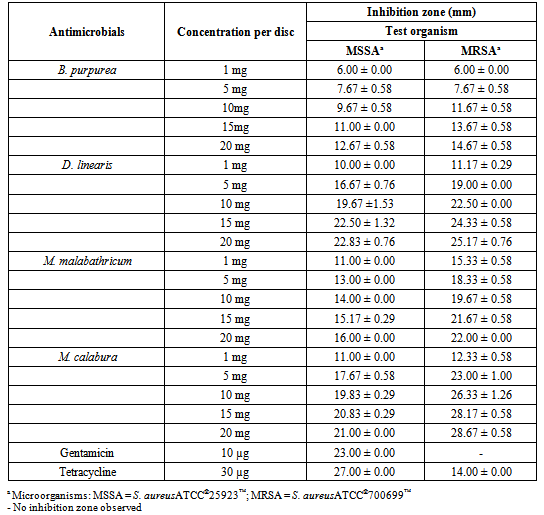
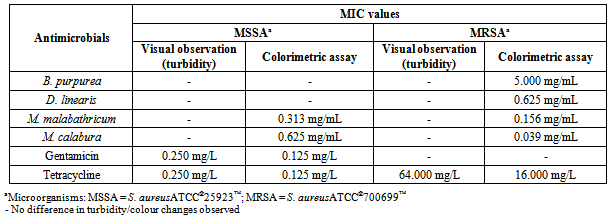
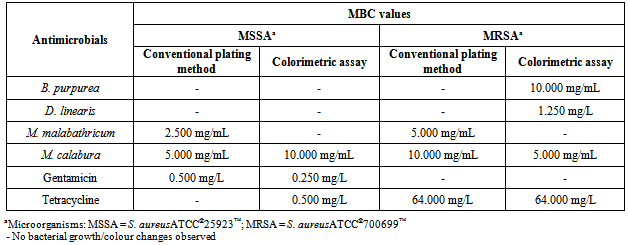
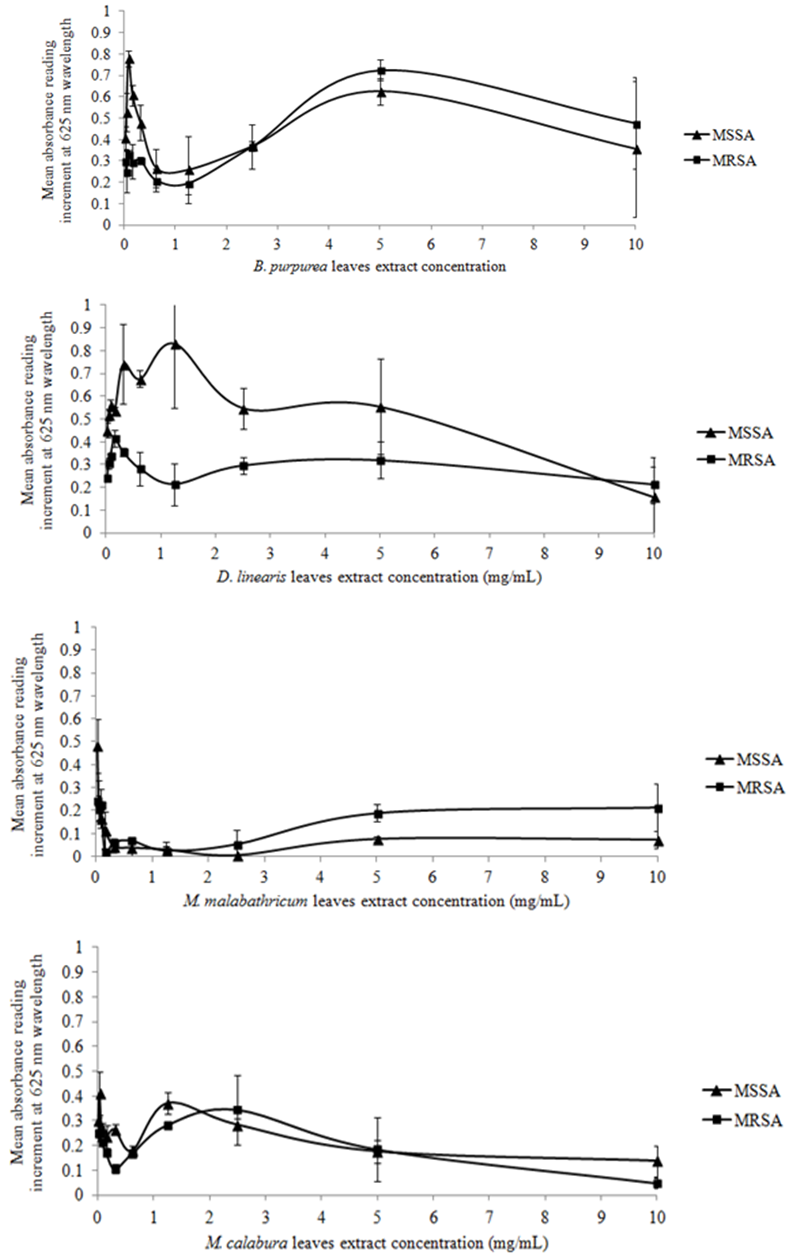
- Table 1 showed the result of preliminary screening of antimicrobial activities of the methanol leaf extracts against MSSA and MRSA via disc diffusion method.The mean absorbance reading increments of MSSA and MRSA treated with four methanol leaf extracts at different concentrations were tabulated in graphs as shown in Figure 1.Table 2 showed the result of the MIC values determined based on colour changes in colorimetric assay, while that for MIC values determination by visual observation was feasible only for the control antibiotics. Table 3 showed the MBC values determined by both conventional plating method and colorimetric assay.
|
|
|
4. Discussion
- The employment of three different methods was an attempt to perform a comparative analyses of the results obtained in this study instead of depending solely on the result of either one of the models, thus increasing the accuracy and dependency of the results, as well as highlighting the feasibility of the methods.Result showed that all four methanol leaf extracts elicited antimicrobial activities as shown by the presence of inhibition zones in disc diffusion method. The antimicrobial effect of all the methanol leaf extracts were found to be dose-dependent as it was observed that the inhibition zoneincreased as the concentration of methanol leaf extract increased. The effect of DMSO was negligible as there was no inhibition of test bacteria growth observed in the presence of 10% DMSO in disc diffusion method. Kruskal-Wallis test also gave a significant difference in supporting the dose dependency of the methanol leaf extracts towards the susceptibility patterns of both respective strains. D. linearis methanol leaf extract elicited the greatest antimicrobial activity against MSSA whereas MRSA showed greatest susceptibility towards M. calaburamethanol leaf extract in disc diffusion method. Interestingly, regardless of the methanol leaf extracts tested, the inhibition zones of MRSA were found to be larger compared to those of MSSA. When the inhibition zones for MSSA and MRSAat each concentration for respective methanol leaf extract were compared, the difference were significant (p < 0.05), signifying the higher antimicrobial activities of all methanol leaf extracts against MRSA than MSSA.It was demonstrated that methanol leaf extracts of D. linearis and M. calabura portrayed higher antimicrobial activities against MRSA, with increment of absorbance reading lower than that of MSSA (as shown by the graph of MRSA which was positioned lower than the graph of MSSA). This finding was supported by the data obtained in disc diffusion method, whereby both D. linearis and M. calaburamethanol leaf extracts elicited greater antimicrobial activities, with the later one elicited the highest antimicrobial activity among all the methanol leaf extracts tested. Vice versa, methanol leaf extracts of B. purpurea and M. malabathricum portrayed lower antimicrobial activities against MRSA (as shown by the graph of MRSA with higher mean absorbance reading increment). This was also supported by the data obtained in disc diffusion method whereby both B. purpurea and M. malabathricummethanol leaf extracts elicited comparably lowerantimicrobial activities than D. linearis and M. calabura methanol leaf extracts. Overall, all methanol leaf extracts tested in this study portrayed antimicrobial activities at different levels against both MSSA and MRSA strains employed in this study. This may give hindsight of their potentials as antimicrobial agents to treat diseases caused by S. aureus. Methanol leaf extracts of D. linearis and M. calabu--ra also showed remarkable antimicrobial activities against MRSA in both disc diffusion and broth microdilution method, therefore suggesting theirefficacies and potentials to develop their bioactive compounds responsible for antimicrobial activities into commercial drugs in treating diseases caused by this life-threatening antibiotic resistant strain.The suspensions of standardized bacterial inoculums with all respective different concentrations of methanol leaf extracts in the broth microdilution method were very cloudy, and that remained throughout the incubation period. This obscured the visual inspection for determining the MIC as the turbidity due to bacterial growth could not be differentiated. Such a limitation rendered the reliability of MIC determinationbased solely on visual observation. Hence, it is necessary to adopt the colorimetric assay to further confirm the MIC values obtained based on visual observation, enhancing the reliability of the results. The MIC values were then determined by results obtained from colorimetric assay. The lowest concentration of methanol leaf extracts used to treat bacterial inoculums which changed the resazurin from blue to purple was considered as the MIC values. The MIC values for all the methanol leaf extracts could only be determined when treated against MRSA. Comparative analysis among the MIC values obtained showed that methanol leaf extract of M. calabura yielded the lowest MIC value compared to other methanol leaf extracts. This was supported by the result in disc diffusion method whereby M. calaburamethanol leaf extract elicited the highest antimicrobial activity among the methanol leaf extracts tested as shown by its largest zone of inhibitions when tested at five different concentrations against MRSA. Complete colour change of resazurin from blue to pink in MSSA bacterial inoculums treated with B. purpurea and D. linearismethanol leaf extracts was observed, indicating non-efficacy of both methanol leaf extracts against MSSA. This was supported by the result from conventional plating methodwhich showed bacterial growth of MSSA on MHA treated with both methanol leaf extracts at all concentrations tested as well as the result from disc diffusion method whereby both methanol leaf extracts elicitedlower inhibition zones compared to M. malabathricum and M. calaburamethanol leaf extracts. Both B. purpurea and D. linearismethanol leaf extracts showed stronger antimicrobial activities against MRSA compared to MSSA as shown by the partial colour change of resazurin, supporting the results in disc diffusion method whereby both methanol leaf extracts formed larger inhibition zones against MRSA than MSSA.Bacterial suspensions treated with M. calaburamethanol leaf extract were the only one which exhibited MBC values using both conventional plating and colorimetric assay methods, converging to its highest antimicrobial activity profile in preliminary screening of antimicrobial activities via the disc diffusion method. MSSA and MRSA treated with M. malabathricum and M. calaburamethanol leaf extracts at various concentrations portrayed MBC values only when tested using the conventional plating method. The colour of resazurin changed from blue to purple or remained aswhen tested using the conventional plating method. The colour of resazurin changed from blue to purple or remained as blue for MRSA treated with B. purpurea and D. linearismethanol leaf extracts at higher concentrations with presence of bacterial growth upon plating on MHA plates. This may signify bacteriostatic phase of the bacterial cells, indicating the MIC values at those extract concentrations. However, this was not the case for MSSA and MRSA treated with M. malabathricum and M. calabura methanol leaf extracts whereby there was a colour change of resazurin from blue to purple without any bacterial growth upon plating on MHA. This could be due to the cytotoxicity of methanol leaf extracts towards the bacterial cells which impaired the cell viability and proliferation. Such impairments reduced the cells' capability to reduce resazurin to resorufin[26] as observed by the partial colour change of resazurin from blue to purple instead of pink. This suggests that the determination of MBC values based solely on the colour change in resazurin may not be convincing enough. It is necessary to employ the conventional plating method in support of the result obtained from colorimetric assay to intensify the accuracy of result.
5. Conclusions
- In conclusion, the demonstrated antimicrobial activities in all the four methanol leaf extracts in this study have given a preface of their potentials as antimicrobial agents. The antimicrobial profiles of all methanol leaf extracts may be due to the presence of bioactive compounds such as flavonoids and phenolic compounds which in previous studies have been found to be effective antimicrobial components against a battery of microorganisms[27-30]. On the other hand, combination of visual observation, optical density measurement and colorimetric assay would provide a better picture of their antimicrobial potentials, as well as identifying the feasibility of the various methods. MBC values would be more practical to be determined by the conventional plating method compared to the colorimetric assay method. This is due to the capability of conventional plating method to render bacterial cells viability observable by unaided eyes by means of bacterial viable growth on the agar plates. Furthermore, the contact of green pigmentation in leaf extracts and blue pigmentation in resazurin caused difficulty in determining the colour change of resazurin post-incubation time, decreasing its feasibility in determining MBC values. Nevertheless, this study attempted a comparison of antimicrobial activities among the four different plants whereby, despite the technical problems as raised earlier, M. calaburamethanol leaf extract seems to be a more promising antimicrobial agent than the other methanol leaf extracts tested. Hence, M. calaburamethanol leaf extract is of particular interest to be further researched and developed as potential antimicrobial agent against S. aureus, specifically the MRSA.
ACKNOWLEDGEMENTS
- The authors are grateful for a research grant (No. 04-01-12-1613RU) provided by UniversityPutra Malaysia (UPM) to support this study.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML