-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Microbiology Research
p-ISSN: 2166-5885 e-ISSN: 2166-5931
2014; 4(1): 1-5
doi:10.5923/j.microbiology.20140401.01
Optimization of Parameters of Biosynthesis of Surface-Active Rhamnolipids by the Strain Pseudomonas sp. PS-17 in the Bioreactor with Injection-Vortex Aeration System
Volodymyr Yerokhin1, Oleksandr Karpenko2, Elena Karpenko1
1Department of Physico-Chemistry of Combustible Minerals, L.M. Lytvynenko Institute of Physical-Organic and Coal Chemistry, NAS of Ukraine, Lviv, 79060, Ukraine
2Department of Technology of Biologically Active Substances, Pharmacy and Biotechnology, Lviv Polytechnic National University, Lviv, 79013, Ukraine
Correspondence to: Oleksandr Karpenko, Department of Technology of Biologically Active Substances, Pharmacy and Biotechnology, Lviv Polytechnic National University, Lviv, 79013, Ukraine.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
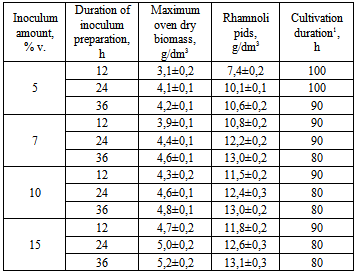
The conditions of the cultivation of the strain Pseudomonas sp. PS- 17 - the producer of surfactants of rhamnolipid nature in the bioreactor with injection-vortex aeration system were studied. The optimal values of some parameters of rhamnolipid biosynthesis were determined: dissolved oxygen concentration, medium pH, as well as the optimum method of inoculum preparation. The efficiency of the use of two-stage temperature control for biosurfactant synthesis process was shown, which increased the accumulation of the product by 14% (from 12,1 to 13,8 g/dm3) and reduced the total cultivation duration on 22 % (from 90 to 70 h).
Keywords: Rhamnolipid surfactants, Biosynthesis, Pseudomonas, Bioreactor, Cultivation parameters
Cite this paper: Volodymyr Yerokhin, Oleksandr Karpenko, Elena Karpenko, Optimization of Parameters of Biosynthesis of Surface-Active Rhamnolipids by the Strain Pseudomonas sp. PS-17 in the Bioreactor with Injection-Vortex Aeration System, Journal of Microbiology Research, Vol. 4 No. 1, 2014, pp. 1-5. doi: 10.5923/j.microbiology.20140401.01.
Article Outline
1. Introduction
- With the development of biotechnology a special attention is drawn to the study of biogenic surfactants (biosurfactants) – products of microbial synthesis. Biogenic surfactants have significant advantages over synthetic ones: they are highly efficient, but at the same time, non-toxic, non-allergenic and biodegradable. The practical significance of microbial surfactants is stipulated by their ability to significantly reduce the surface and interfacial tension of aqueous solutions, their emulsifying, foaming properties and environmental safety[1]. Inexpensive substrates, including waste production can be used for the synthesis of microbial surfactants[2]. Despite the fact that biosurfactants are a relatively new biotechnology product, the expediency of their use in the oil, paper, wood and paper, food, textile, perfume and cosmetics, pharmaceutical industries as well as environmental protection and agriculture was grounded[3].One of the most promising classes of biosurfactants are rhamnolipids – products of biosynthesis of bacteria of the genus Pseudomonas[4]. Currently the technologis of rhamnolipid products are not widely used in industry, primarily due to the low yield of the final product and excessive foaming during fermentation. But given the current world trends of transition to environmentally friendly products, the development of industrial technology of biosynthesis of rhamnolipids is especially topical. Development of an efficient technology of synthesis of surface-active rhamnolipids can be realized primarily as a result of the determination of optimal conditions for culturing the strains-producers and application of new energy-efficient approaches to the process of biosynthesis and the isolation of target products[5].Production of biosurfactants depends on many factors: the effectiveness of the producer strain, culture medium composition, temperature, pH, aeration, duration of fermentation, bioreactor type and method of cultivation. Previously it was shown that a strain Pseudomonas sp. PS- 17 is a promising producer of rhamnolipid surfactants[6]. The optimum composition of the nutrient medium for biosynthesis of rhamnolipids was determined[7]. It is shown that to achieve high yield of rhamnolipids the most effective construction of bioreactor is the one with injection-vortex aeration system, which also allows the control of the foaming process in the biosynthesis of surfactants[8]. In this connection, the aim of this work was to develop an effective technology of biosynthesis of rhamnolipids by the strain Pseudomonas sp. PS- 17 in a laboratory fermenter - namely, the determination of the optimum parameters of aeration, temperature, pH and inoculum.
2. Materials and Methods
- The bacterial strain Pseudomonas sp. PS-17 – producer of extracellular surfactants – rhamnolipids from the Collection of Cultures of Department of Physico-Chemistry of Combustible Minerals L.M. Lytvynenko In POCC NAS of Ukraine was used for studies. Cultivation of the strain was performed in the nutrient medium with the following composition (g/dm3): glycerol – 30, sodium citrate – 4,0; NaNO3–3,0; K2HPO4×3H2O–2,0; KH2PO4–1,2; MgSO4 × 7H2O – 0,5[7].The fermentation was carried out in the laboratory bioreactor with injection-vortex aeration system and volume of 5 dm3. Sterilization of the fermenter was performed with nutrient medium for 45 min at 125ºC.Cultivation was carried out at medium pH 6, 7, 8, 9 or without pH adjustment. Maintainance of the desired pH value was performed via the addition of 1 N solutions of NaOH and HCI. Fermentation temperature – 26, 28, 30, 32, 34, 36ºC – was maintained via external thermostating coil in the shell of the reactor.The following parameters were controlled in the process of the fermentation: biomass amount, concentration of rhamnolipids, pH of the medium, temperature, dissolved oxygen content in the culture liquid. The values of pH, temperature and dissolved oxygen concentration were determined using an automatic analyzer “Expert-001-4.0.1” (LLC “Ekonyks-Expert”, Russia) in real time conditions.Aeration of the culture medium was carried out using a compressor (SERA air550R, “Sera”, Germany) at the speed of 1 m3/m3∙min, which was controlled by a laboratory rotameter. The concentration of dissolved oxygen was controlled by adjusting the velocity of culture fluid flow through the injector using a peristaltic pump.The amount of bacterial biomass was determined by optical density on a spectrophotometer UVmini - 1240 ("Shimadzu", Japan) at a wavelength of 540 nm in cuvettes 5 mm[9].Rhamnolipids concentration in the culture liquid was determined with orcinol method using a spectrophotometer UVmini - 1240 ("Shimadzu", Japan)[10].The biomass productivity Yp/x for rhamnolipids was defined as the ratio of rhamnolipid output (g/dm3) to a biomass concentration (g ODB/dm3) and expressed in g/g ODB.Output of surfactants from the substrate was determined as the ratio of rhamnolipid concentration in culture liquid (g/dm3) and substrate concentration (g/dm3) and expressed as g/g of substrate.
3. Results and Discussion
- In our previous studies it was shown that the most effective bioreactor for the cultivation of the strain Pseudomonas sp. PS-17 is a fermenter with injection-vortex aeration system. It was shown that for the effective synthesis of the target products it is necessary to ensure the supply of oxygen into medium in amount Ks = 1 kg O2/(m3∙h). Such mass transfer conditions are provided in the fermenter with injection-vortex aeration system, which is a reactor without reflecting walls with bottom-drive turbine stirrer equipped with an external circulation loop with injector for supplying air[8]. At that, only the rate of oxygen saturation of the culture medium was taken into account, and the change in the concentration of dissolved oxygen in the cultivation has not been studied. In fact, the latter parameter is important because it allows the control of aeration efficiency and prevention of the inhibition of the growth of culture and synthesis of the target product. Therefore, our objective was to study the changes in the concentration of dissolved oxygen in the culture medium during fermentation of the strain Pseudomonas sp. PS- 17. The dynamics of dissolved oxygen content when culturing the strain in a fermenter with injection-vortex aeration system is shown in Figure 1.
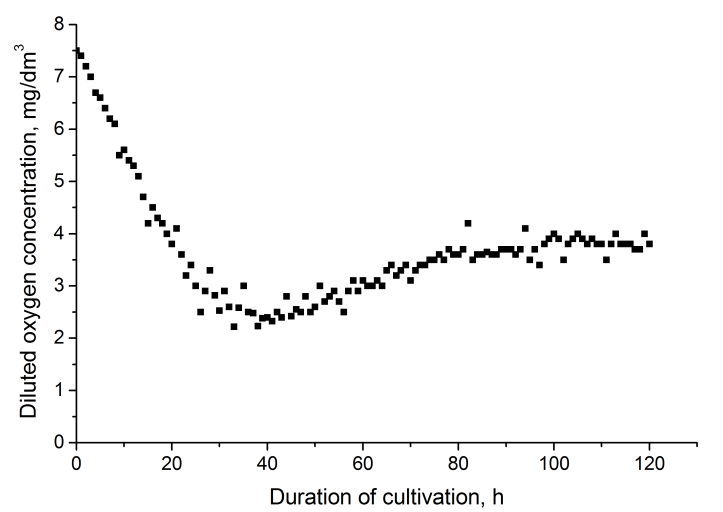 | Figure 1. Change in the concentration of dissolved oxygen in culture medium when cultivating the strain Pseudomonas sp. PS-17 in injection-vortex bioreactor |
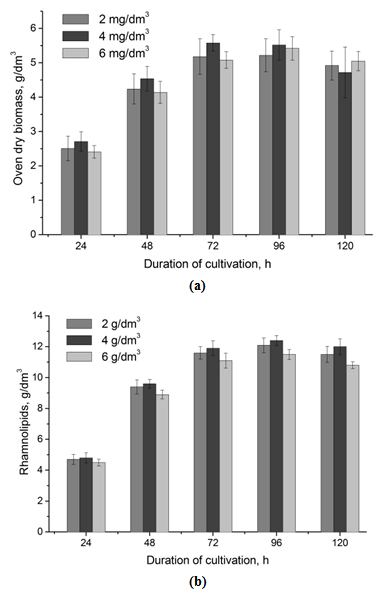 | Figure 2. The growth of the strain Pseudomonas sp. PS-17 (a) and accumulation of rhamnolipids (b) depending on the concentration of dissolved oxygen in the medium |
|
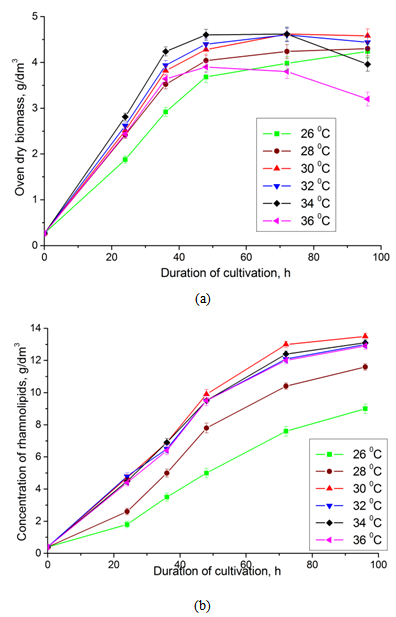 | Figure 3. Dynamics of growth (a) and accumulation of rhamnolipids (b) by the strain Pseudomonas sp. PS-17 at different temperatures of cultivation |
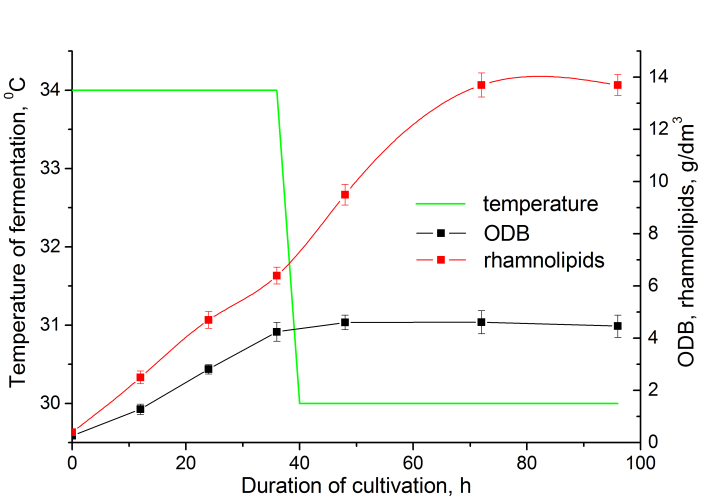 | Figure 4. The growth and synthesis of biosurfactants by the strain of Pseudomonas sp. PS-17 when applying two-stage temperature regime |
4. Conclusions
- It was determined, that biomass accumulation and synthesis of rhamnolipids by the strain Pseudomonas sp. PS-17 in the injection-vortex fermenter doesn’t depend on the dissolved oxygen content in culture liquid if the latter parameter is in the range 2-6 mg/dm3.It was shown that for the production of inoculum optimum it is reasonable to cultivate the strain for 36 h at 34°C at pH 7,0. Optimum concentration of inoculum – 7% vol. (viable titer - 2×108 CFU/cm3) in medium. The efficiency of the application of two-stage temperature regime for the process of biosurfactant synthesis was shown: for the first 36 h temperature was set to 34°C, then (during 4 h) – a gradual decrease in temperature to 30°C. This mode of fermentation allowed the increase of culture productivity and accumulation of the product to 13,8 g/dm3, as well as reduction of the process duration on 12,5% (to 70 h).It was determined that the maximum accumulation of rhamnolipids by the strain Pseudomonas sp. PS-17 is achieved without pH adjustment (pH 8,2-8,4).
ACKNOWLEDGEMENTS
- The heading of the Acknowledgment section and the References section must not be numbered.SAP Productions wishes to acknowledge all the contributors for developing and maintaining this template.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML