-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Microbiology Research
p-ISSN: 2166-5885 e-ISSN: 2166-5931
2013; 3(6): 266-273
doi:10.5923/j.microbiology.20130306.12
Low Level of Pre-Vaccination Measles Antibody among Infants Receiving Measles Immunization in Ilorin, Kwara State, Nigeria
Fowotade A.1, Okonko I. O.2, Nwabuisi C.3, Fadeyi A.3, Bakare R. A.1, Adu F. D.4
1Department of Medical Microbiology & Parasitology, University College Hospital, Ibadan, Nigeria
2Medical Microbiology Unit, Department of Microbiology, University of Port Harcourt, P.M.B. 5323, Choba, East-West Road, Port Harcourt, Nigeria
3Department of Medical Microbiology & Parasitology, University of Ilorin Teaching Hospital, Ilorin, Nigeria
4Department of Virology, College of Medicine, University of Ibadan, Ibadan, Nigeria
Correspondence to: Okonko I. O., Medical Microbiology Unit, Department of Microbiology, University of Port Harcourt, P.M.B. 5323, Choba, East-West Road, Port Harcourt, Nigeria.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
This study was designed to assess the low levels of pre-vaccination measles antibody among infants receiving measles immunization in Ilorin, Kwara State, Nigeria. Pre-vaccination blood samples were obtained from 400 infants brought to the EPI Clinic of the University of Ilorin Teaching Hospital, Ilorin, Nigeria. Blood samples were collected by finger puncture onto ROPACO (Rochester, USA) rectangular filter paper measuring 7 cm by 10 cm. Other information like name, age, sex, date of vaccination was also recorded on the filter paper. The filter papers were dried at ambient temperature and stored in plastic bags at -20℃ until ready for serum extraction. Specimens were analyzed for measles antibody using Hemagglutination Inhibition technique. Data generated were subjected to Chi square statistical test to establish association between categorical variables with dichotomous outcomes. Of all the 400 infants screened for pre-vaccination measles antibodies, 29(7.2%) had protective antibody titer while 156 (39.0%) had low titer since ≥40 HI titer is the study threshold of protection against measles while 215(53.8%) had no detectable measles antibody titer (<1:10). Thirty-one (8.0%) of the infants had measles prior to vaccination. Previous history of measles was significant associated (P=0.0005, X2 = 34.507) with the level of pre-vaccination measles virus antibody. There was no significant difference (p=0.723) in the level of measles virus antibody among the exclusively breastfed infants and those that were not exclusively breast fed. Age (p=0.839) and sex (p=0.1999) were not significantly associated with the level of measles virus antibody. The distribution of this antibody levels appeared stable with no significant difference between age groups, sex and breast feeding pattern. Only the previous history of measles showed statistical association with measles virus antibody proportion. In conclusion, a larger proportion (53.8%) of the children had no detectable anti-measles virus antibody while 39.0% had low (non-protective) titer; these put both groups at risk of developing measles given the endemic nature of Nigeria. Therefore, re-evaluation of 9 months as the age for measles vaccination in Nigeria vis-à-vis 92.8% with <40 HI titer is highly recommended.
Keywords: Hemagglutination Inhibition, Pre-vaccination, Measles Antibody, Measles Immunization, Vaccination
Cite this paper: Fowotade A., Okonko I. O., Nwabuisi C., Fadeyi A., Bakare R. A., Adu F. D., Low Level of Pre-Vaccination Measles Antibody among Infants Receiving Measles Immunization in Ilorin, Kwara State, Nigeria, Journal of Microbiology Research, Vol. 3 No. 6, 2013, pp. 266-273. doi: 10.5923/j.microbiology.20130306.12.
Article Outline
1. Introduction
- Measles infection occurs worldwide but it is epidemic in developing countries where severe morbidity and high mortality are associated with underlying malnutrition, poverty, and indiscriminate vaccination services[1- 3]. The optimum age for measles vaccination varies from country to country thus a standardized vaccination schedule is controversial. While the increase in measles vaccination coverage has produced significant changes in the epidemiology of infection in developing countries, measles morbidity and mortality represents an important public health problem, with a significant number of infections occurring earlier than the 9 months[4]. Several researchers have reported the occurrence of measles in infants less than 9 months old[5-6]. Some studies have attributed the occurrence of measles among infants less than 9 months to low affinity maternal measles antibody which consequently increases the vulnerability of these infants to circulating wild measles strains[7-8]. Oyedele et al.[6] revealed that while 58% of Nigerian children lost their protective maternal antibody by the age of 4 months only 3% had enough antibodies to protect them between the ages of 6-9 months.Nevertheless, in West Africa, measles vaccine is given at 9 months of age which based on an early serological study in Kenya is thought to be the optimal age for protection against measles in endemic areas where maternally derived antibody as a result of natural measles may inhibit the vaccine[8]. Since then as coverage has increased and the majority of mothers are now vaccinated which has resulted in lower concentrations of measles antibody transferred to their infants and a lower age of susceptibility to measles[9]. Surprisingly, there have been few papers from sub -Saharan Africa which have documented this phenomenon even though it is known that during outbreaks of measles the incidence of measles and mortality is high in young infants. An implication of these observations was that lowering the age of measles vaccination might be beneficial[9].In Nigeria, infants are vaccinated against measles at the age of 9 months. However, persistence of maternal antibodies has been correlated with vaccination failure among infants less than a year of age, thereby making a mockery of vaccination of infants under the age of one year[10, 11]. Vaccine- induced immunity also appears to be shorter lived than one engendered by wild virus, suggesting that infants of vaccinated mothers may lose passively acquired antibodies faster than those of previously infected mothers[6]. A considerable number of cases of measles occur during the first year of life although overt measles before the age of 6 months is rare because of the supposed protection provided by the maternal antibodies[12]. Measles takes a serious course and may lead to death in children between 1-5 years and factors not yet identified converge to give this effect[12, 13]. Infection with wild measles virus causes extensive mobilization of the immune defenses[12]. Subclinical infections in non-immunity such as maternal antibodies or active immunity may have virus replication in the body cause mild or no clinical symptoms[12]. After a regular infection, Black and Rosen[14] showed that titers of antibodies be demonstrated throughout life in essentially all cases even in the absence of re-exposure[12].According to Odomele et al.[12], development of immunity to this disease is either congenital, vaccination or infection by wild type. The sources of maternally transmitted antibodies in children are the antibody transferred in utero via the placenta and the colostrum (breast milk)[12]. The probability of this transmission is a function of complexity and class of the antibodies[12].Several studies showed in utero transmission as the only source by which mothers transmit antibodies to their children[12] while Adu and Adeniji[15] showed children derive maternal antibodies from breast milk when sucking. Placental transfer of immunoglobulin antibodies is a function of complexity and class of the antibody. The role of maternal antibodies in the seroconversion to measles vaccination is documented[12, 15].Thus, this paper touches on an important and under researched topic. This study aimed at determining the level of low pre-vaccination measles antibody among infants receiving measles immunization in Ilorin, Kwara State, Nigeria. It also evaluated the prevalence of low pre-vaccination measles antibodies in the sera of children brought for measles vaccination and established the rate of seroconversion to measles vaccination in children of nursing mothers. It also evaluated the potency of the vaccine in relation to seroconversion.
2. Materials and Methods
2.1. Study Population
- Ethical consideration was sought and obtained from the UITH Ethical Research Committee. Pre-vaccination blood samples were obtained from 400 infants brought to the EPI Clinic of the University of Ilorin Teaching Hospital, Ilorin, Kwara State, Nigeria.
2.2. Sample Collection
- Blood samples were collected by finger puncture onto ROPACO (Rochester, USA) rectangular filter paper measuring 7 cm by 10 cm as described by Nakano et al.[16]. Other information like name, age, sex, date of vaccination was also recorded on the filter paper. The filter papers were dried at ambient temperature and stored in plastic bags at -20℃ until ready for serum extraction.
2.3. Collection of Epidemiological Data
- Age, sex, breast feeding pattern and previous history of measles were collected using a Performa specially designed for this study.
2.4. Source of Antigen
- The antigen used, was obtained from the measles vaccine collected from the National Program on Immunization unit (NPI) of University of Ilorin Teaching Hospital, Ilorin, Kwara State, Nigeria. The Measles vaccine which is live attenuated (Freeze-dried) is prepared from the Edmonston strain of measles virus attenuated by twenty-two passages on human diploid cells (HDC) and is known as the Edmonston – Zagreb strain. The lyophilized vaccine is provided with diluents and the vaccine meets the requirement of WHO when tested by the method outlined in WHO requirement for vaccine production[17, 18].
2.5. Potency of the Vaccine
- Each single human dose of the vaccine contains 1000CCID50 of live virus particles when reconstituted in a volume of 0.5ml. Stability data shows that the vaccine retains the potency of 1000CCID50 per dose after 1 week at 37ºC[17-20].
2.6. Cultivation of the Antigen
- Measles antigen was prepared by inoculation of Edmonston strain of measles vaccine on Vero/SLAM cells (106cells/ml). The serum titer was expressed as the highest dilution of the serum showing complete inhibition of hemagglutination. A grown monolayer of Vero/SLAM cell in a tissue culture bottle obtained from the tissue culture unit of World Health Organization National Polio Laboratory, Department of Virology, University of Ibadan, Ibadan, Nigeria was used for the inoculation of the vaccine antigen. After 72hrs, maximum development of the CPE was observed using an inverted microscope. The tissue culture growth was then frozen and thawed three times to reduce the virulence of the virus. The measles virus culture was then centrifuged at 3000rpm for 20 minutes using a refrigerating centrifuge to obtain suspension of the viral particles which were then dispensed in aliquots of 1mls into cryovials, and stored in the freezer, ready for use[17-20].
2.7. Susceptible Erythrocyte
- The susceptible erythrocyte (Red blood cell) was used as described by Hsiung and Fong[21].
2.8. Vaccine Titration
- The vaccines were titrated as previously described by Onoja et al.[22]. The vaccine titer was calculated by the method of Reed and Muench[23].
2.9. Serological Analysis
- Haemagglutination inhibition test (HI) was used to measure the measles antibodies as described by Brooks et al.[24] and the resulting titers from the various assays are as contained in the table of results. About 0.025ml of 0.05ml of 1:10 dilution of sera was mixed each with 0.025ml of PBS/BSA (pH 7.2) of the 4HA (haemagglutinating) units of commercially available measles antigen. This gave a range of serial two-fold dilution of 1:10 to 1:256, except the controls. The plates were shaken and mixture incubated at 37OC for one hour. About 0.05ml of 1% washed AGM-RBC was added and kept at 37℃ until the cells in the control wells had settled at the bottom before results were read. The serum HI titer was expressed as the highest dilution of the serum showing complete inhibition of haemagglutination. Measles virus antibody ≥40 HI titer is the study threshold of protection against measles. Measles virus antibody of <1:10 HI titer was considered to be undetectable while 1:10 to 1:20 HI titer was considered to be low titer.
2.10. Data Analysis
- Identification of factors that may correlate with the seroconversion was carried out by the Chi-square (X2) distribution method using Microsoft Excel. Confidence interval was set at p< 0.05. In the analysis, I used <1:10 as negative and ≥ 1:10 as positive in order to get valid inferential analysis.
3. Results
- The three vials of vaccine strain of measles virus cultured in Vero/SLAM cells had virus titer values of 3.5, 3.25 and 3.5 Log which is according to the WHO minimum standard of 3.0Log (1000CCID50) per human dose.
3.1. Prevaccination Measles Antibody Titer
- Of all the 400 infants screened for pre-vaccination antibodies, 371/400(92.8%) had measles virus antibody titer which ranged from <1:10 to 1:20 while the remaining 29/400 (7.2%) had measles virus antibody titer of >1:40. It showed that 215(53.7%) children of all ages had no detectable prevaccination antibodies titer.
3.2. Sex Distribution of Infants
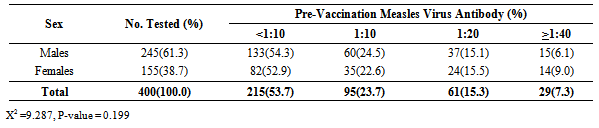
- A total of 400 infants were recruited into the study over the period of 12 months. Among the pre-vaccination group, there were 245/400(61.3%) males and 155/400(38.7) females, giving a male to female ratio of 1.6: 1.
3.3. Age Distribution of Infants
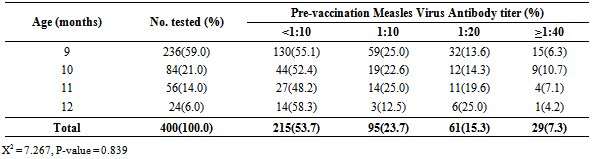
- The age distribution of the infants recruited into the study were as follow; the majority, 236/400 (59.0%) were aged 9 months, 84/400 (21.0%) were aged 10 months. The others were 56/400 (14.0%) eleven month olds and 24/400 (6.0%) twelve month old infants (Table 1).
3.4. Measles Virus Antibody Titer According to Age
- Among the 9 month old infants, only 15/236(6.4%) still had high measles antibodies of 1:40 pre-vaccination, 91/236 (38.6%) had low maternal antibodies which ranged from 1:10 to 1: 20 while the remaining 130/236 (55.1%) had no detectable measles virus antibody titer as shown in Table 1. Children within 10 years of age had higher prevalence of prevaccination antibody titer (>1.40) while children 12 years of age had higher prevalence of no detectable prevaccination antibody titer. However, there is no significant difference in the level of pre-vaccination antibody titer among the various age groups (X2 = 7.267, P-value = 0.839).
|
3.5. Measles Virus Antibody Titer According to Sex
- Among the male infants, 133/245(54.3%) had pre-vaccination measles virus antibody titer of < 1:10, 60/245 (24.5%) had antibody titer of 1:10, 37/245 (15.1%) had titer of 1:20, while the remaining 15/245(6.1%) had antibody titers of >1:40. In the same vein, 82/155 (52.9%) females had no detectable antibody titer, 35/155(22.6%) had antibody titer of 1:10, 24/155 (15.5%) had titer of 1:20, while 14/155 (9.0%) had titer of >1:40 (Table 2).
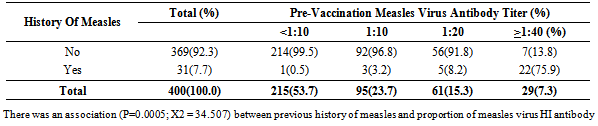
3.6. Impact of Previous Measles Infection on Pre-vaccination Antibody Titer
- Only 31/400(7.8%) of the vaccinees had previous history of measles prior to vaccination while the remaining 369/400 (92.2%) had no history of measles. Twenty two (70.9%) of the 31 vaccinees with previous history of measles had pre-vaccination measles virus antibody titer of >1:40. Of the 369 infants without a previous history of measles, only 7(1.9%) had protective pre-vaccination antibody titer (Table 3). There was significant difference in the level of pre-vaccination measles virus antibody among the group that had previous history of measles (X2 = 34.507, P-value = 0.0005).
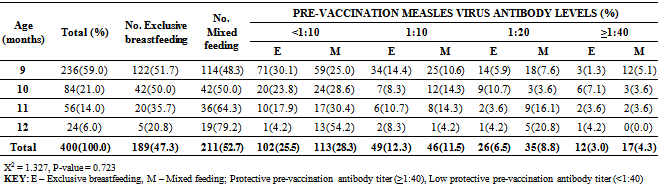
3.7. Impact of Exclusive Breastfeeding on Pre-vaccination Antibody Titer
- All the 400 infants were breastfed. A total of 189 (47.3%) infants were breast fed exclusively while 211(52.8%) were non-exclusively breast fed (Table 4). Twelve (6.3%) of the 189 that were exclusively breast fed had protective pre-vaccination antibody titer, while 17 (8.1%) of the 211 non-exclusively breast fed infants had protective antibody titer (Table 4). There was no significant difference in the level of pre-vaccination antibody titer among the infants that were exclusively breast fed and those that were not (X2 = 6.617, P-value = 0.470)
4. Discussion
- The study showed that of all the 400 infants screened for pre-vaccination antibodies, 92.8% had low pre-vaccination measles antibody titer which ranged from <1:10 to 1:20 while the remaining 7.2% had protective antibody titer of >1:40. It also showed that the lowest pre-vaccination antibodies titer was 4 and the highest titer was 256. This HI antibodies clusters agrees closely with the report of Deepti et al.[25], Akyala et al.[19], Motayo et al.[26] and Ogundiji et al.[27]. This agreement may be due to the same haemagglutination inhibition serological technique employed[19, 26, 27]. The Haemagglutination inhibition (HI) test is the most widely acceptable test in most developing countries like Nigeria[26]. HI test has long for a long time a gold standard for laboratory diagnosis of measles infection [26]. This is because it is very sensitive and specific and easily performed in any laboratory[26].
|
|
|
ACKNOWLEDGEMENTS
- Our deep and sincere gratitude goes to all those who voluntarily participated in this study. The authors would like to thank management and staff of World Health Organization Regional Reference Laboratory for Polio, Department of Virology, College of Medicine, University of Ibadan for their support throughout the period of this study. The excellent technical assistance of Dr. Bola Oyemankinde, Miss Olufunke Adewale and Mr. Oluwaseun Adedeji among others is sincerely acknowledged.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML