-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Microbiology Research
p-ISSN: 2166-5885 e-ISSN: 2166-5931
2013; 3(6): 261-265
doi:10.5923/j.microbiology.20130306.11
Comparison of Lyme Disease Prevalence and Disease Reporting in an Endemic Area
Holly Ahern
Biology Department, State University of New York at Adirondack, Queensbury, NY, 12804, USA
Correspondence to: Holly Ahern, Biology Department, State University of New York at Adirondack, Queensbury, NY, 12804, USA.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Lyme disease is the most commonly reported tick-borne illness in the United States; however, controversy surrounding the diagnostic criteria has led to claims of both under-diagnosis and over-diagnosis of Lyme disease by physicians. While both result in errors in estimating disease risk, under-reporting of Lyme disease to public health agencies underestimates the risk and increases the disease burden on individuals and society. A population based cross-sectional study was conducted to evaluate the rate of “Probable” Lyme disease diagnosed according to CDC criteria. Responses were compared to electronic Lyme disease surveillance statistics. The survey had a response rate of 60% (n = 600). Two percent of the survey respondents reported being diagnosed with Lyme disease according to CDC criteria for “Probable” Lyme disease, which is significantly higher than the number of reported cases. Sixteen percent of undiagnosed survey respondents reported subjective signs and symptoms consistent with “late-stage” Lyme disease. Thus, in a region endemic for Lyme disease, cases are diagnosed by physicians more frequently than cases are reported. Additionally, a significant proportion of the study population reported signs and symptoms consistent with late-stage Lyme disease. Together, these results indicate underestimation of Lyme disease risk and an increase in public health burden for people living in endemic areas.
Keywords: Lyme disease, Burden of disease, Surveillance case definition, Tick-borne disease
Cite this paper: Holly Ahern, Comparison of Lyme Disease Prevalence and Disease Reporting in an Endemic Area, Journal of Microbiology Research, Vol. 3 No. 6, 2013, pp. 261-265. doi: 10.5923/j.microbiology.20130306.11.
1. Introduction
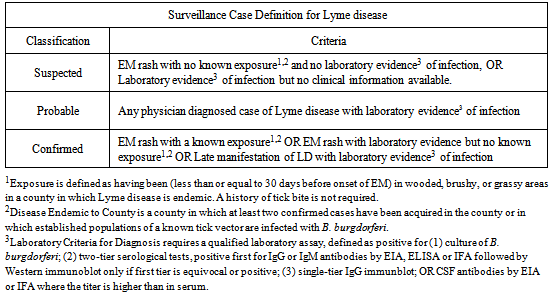
- Lyme disease is a multi-system infectious disease transmitted by ticks. According to public health surveillance data, Lyme disease is the most commonly reported vector-borne illness in the United States, ranking as the 7th most common Nationally Notifiable disease in 2012. The Centers for Disease Control (CDC) reports that residents of 13 states account for 96% of reported Lyme disease cases [1]. Lyme disease is, however, widely considered to be under-reported, both in highly endemic regions of the United States[1, 2, 3, 4, 5] as well as areas where Lyme disease is not thought to be endemic[6, 7, 8, 9]. For public health purposes, determination of disease prevalence and estimation of risk depends on a physician’s ability to make a diagnosis, along with case reporting compliance. A surveillance case definition developed by the Council of State and Territorial Epidemiologists (CSTE) is currently used by the Centers for Disease Control (CDC) for Lyme disease reporting in the United States[10]. The CSTE criteria for surveillance case reporting are shown in Table 1. The most widely accepted guidelines for physician diagnosis of Lyme disease are based on the CDC surveillance criteria and rely primarily on clinical findings. An algorithm devised to facilitate physician diagnosis of Lyme disease includes case definition criteria and advises physicians that patients who show no objective signs have a low probability of Lyme disease[11]. Only two objective clinical signs are considered reliable indicators of infection by Borrelia burgdorferi: an erythema migrans (bulls-eye) rash of at least 5 cm in diameter at the site of a tick bite, and “laboratory evidence” of infection, which is described in Table 1. According to the CDC, a majority (60-80%) of patients with Lyme disease develop the “bulls-eye” rash which substantiates its use as a clinical sign of infection[2]. However, in practice the classic EM rash is only rarely the predominant morphologic lesion, with other types of rashes or no rash occurring in over 50% of cases[12, 13, 14]. The EM rash was also shown to appear only following bites from nymph stage, but not adult stage, ticks.[15]. Thus, a patient presenting with a rash other than EM, no rash because they were bitten by an adult tick, or with signs and symptoms of Lyme disease but with no laboratory evidence or evidence of exposure, would not be considered a “case” of Lyme disease according to the current criteria. Laboratory evidence for Lyme disease includes a positive culture of Borrelia from a clinical sample, or detection of serum or CSF antibodies for B. burgdorferi. Serological tests are reported to have high specificity, but are of low sensitivity, particularly in the early and late stages of the infection[16]. Also, there is significant variation in the interpretation of Western immunoblots in terms of which antibody response (IgG or IgM) produces more reliable results, which “bands” are indicative of infection with B. burgdorferi, and which reference lab performs and interprets the test[17, 18].
|
2. Method
- To evaluate the frequency of diagnosed and undiagnosed cases of Lyme disease in an endemic region of New York State, a cross-sectional study based on a survey instrument was conducted. The population studied consisted of students, faculty and staff at a non-residential community college in upstate New York. This group was presumed to represent a reasonable cross section of the populations of the three counties (Saratoga, Warren, and Washington) served by the college. The survey was based on self-reporting by respondents and was not limited to any specific group. The data was analyzed without regard to the age or gender of the respondents, or presence or absence of any other disease state.The survey was designed with two parts. Part A of the survey asked respondents to report if they had been diagnosed with Lyme disease by a physician, and if diagnosed were asked to further report if they had a “positive blood test,” which is reportable as “Probable” Lyme disease New York. Requirements for positive blood test were not further defined. Part A of the survey also asked diagnosed respondents if they recalled a tick bite or a “bulls-eye” rash, if they were treated with antibiotics, and if their Lyme disease symptoms were gone. Part B asked undiagnosed participants to choose from a list of cardiac, rheumatologic, and neurological signs and symptoms that are consistent with late-stage Lyme disease. These were drawn from previously published studies[23] and the CDC description of “late manifestations” of Lyme disease[1, 12]. A scoring system was established such that symptoms most closely associated with Lyme disease were scored higher than those that were more generalized. Prior to the administration of the final survey, a preliminary instrument was provided to a group of people diagnosed with Lyme disease according to CDC criteria (positive two-tier laboratory test). A threshold value was established and respondents whose score met or exceeded the threshold were counted as having signs and symptoms consistent with late-stage Lyme disease. The instrument was revised and validated through administration to another group of Lyme patients. The final survey was administered over the course of one week in April 2010 to a random sample of the community college population. Data was entered into an Excel spreadsheet and analyzed for prevalence rates using 2010 United States Census Bureau data relevant to Saratoga, Warren, and Washington Counties in New York State. Surveillance case rates for Lyme disease were determined using electronic records obtained from the New York State Department of Health (NYSDOH).
3. Results
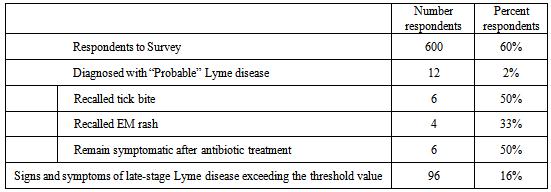
- The survey had a response rate of 60%. Among survey respondents (n=600), 12 (2%) reported that they had been diagnosed with Lyme disease by a physician with a positive blood test, therefore meeting the CDC criteria for “Probable” Lyme disease. Applying that rate to the population of the three counties obtained from US Census Bureau statistics for that year (348,530) infers that close to 7,000 people in those counties had been diagnosed with Lyme disease at the time of the survey. By comparison, for the three counties considered in this study, there were a total of 1,930 confirmed cases of Lyme disease reported for the years 2002-2011. Specifically for the year 2010, there were a total of 462 cases of Lyme disease reported for the entirety of New York State excluding New York City[24]. Of interest is that only half (50%) of the diagnosed respondents also reported that they remembered the tick bite, and only 33% reported appearance of an erythema migrans (bulls-eye) rash at the site of a tick bite. Additionally, half of the diagnosed respondents that had been treated with antibiotics reported persisting symptoms and did not consider themselves “cured.” For part B of the survey, 96 (16%) of the undiagnosed respondents met the threshold value for possible Lyme disease, indicating that they were experiencing cardiac, rheumatologic, or neurological symptoms consistent with late-stage Lyme disease (Table 2).
|
4. Discussion
- Based on a system of self-reporting by respondents in a community setting, the results of this study indicate that there is considerable disparity between the rate of Lyme disease diagnosis by physicians and the reported number of surveillance cases. Factors that might contribute to underreporting of Lyme disease include misdiagnosis of Lyme disease as other disorders with overlapping symptoms (such as fibromyalgia, chronic fatigue syndrome, or multiple sclerosis); lack of a diagnosis due to patients not presenting with either of the two accepted markers for the disease (EM rash or laboratory evidence); poor recognition or understanding of the signs and symptoms of disseminated Lyme disease; diagnosis of Lyme disease by physicians who then don’t report the cases; the case report being discounted by public health agencies as not fully meeting the CDC criteria for a case of Lyme disease; or reluctance on the part of the physician to report the case. A survey of primary care physicians in Connecticut, in which over half of the respondents reported uncertainty about Lyme disease diagnosis, is reflective of the problems with the current diagnosis and reporting system[25].Self-reporting of symptoms consistent with “late-stage” Lyme disease in Part B of the survey by undiagnosed respondents showed a considerable number of people with signs and symptoms that are highly consistent with what is termed “late-stage” Lyme disease. The majority of people who self-reported these symptoms did not indicate if they had been diagnosed with any other disease, therefore it is not possible to conclude that these respondents had Lyme disease. However, it should be considered that because these respondents live in a region known to be highly endemic for a disease to which their symptoms match, there is a likelihood that their symptoms may in fact be due to undiagnosed Lyme disease. The results of this study are consistent with the outcomes of similar studies conducted in other parts of the country[5, 6, 8] in which Lyme disease cases were reported to public health agencies at rates far below the rate of diagnosis. A project conducted by a team of medical students at the University of Massachusetts demonstrated high rates of Lyme disease on Martha’s Vineyard through field surveys conducted on ferries, schools, and churches in conjunction with interviews of health care workers and pharmacists. While there were over 1,000 prescriptions written for doxycycline as treatment for Lyme disease, only 25 cases of Lyme disease were recorded by the CDC for Dukes County in 2010, indicating that doctors diagnosed and prescribed antibiotics for Lyme disease at a rate 50X higher than the number of reported cases[5].The data obtained from the cross-sectional survey in this study is also consistent with the preliminary results of a series of investigational surveys conducted by the CDC, leading the CDC to revise upward their estimate of the number of cases of Lyme disease in the US from approximately 30,000 to 312,000 in 2012, a factor of 10X[26].While Lyme disease is considered by the CDC to be “highly localized,” a prevalence survey completed in a non-endemic area (Georgia) with a low reported incidence for Lyme disease revealed that the rate of Lyme disease diagnosed by family physicians both clinically and by serologic test was 40X higher than the reported prevalence of the disease[9]. This conclusion was also reached in an investigation of morbidity associated with insect bites in South Carolina, where the rate of diagnosis of Lyme disease was 15X higher[7] and in Maryland where Lyme cases were reported 10 to 12X less often than diagnosed[6]. Most recently, human Lyme borreliosis was shown by PCR and DNA sequencing to occur Florida and Georgia, with the implication that some cases of a Lyme-like illness in the southern US referred to as southern tick associated rash illness (STARI) may be attributable to previously undetected B. burgdorferi sensu lato infections[27].Prevalence studies using companion animals as sentinels for Lyme disease strongly suggest that Borrelia antibody seroprevalence in dogs correlates to reported human incidence[28, 29]. A canine seroprevalence study conducted by the Vermont Department of Health in 2010 demonstrated that 16.08% (averaged over 13 counties) of the dogs in Vermont tested positive for Borrelia antibodies[30] This correlates almost exactly to the rate of disease symptoms experienced by survey respondents (16%). The three counties in Vermont with the highest canine seroprevalence of Lyme disease, Addison (23.85%), Bennington (23.17%), and Rutland (28.73%) border the area of New York State investigated in this study. A potential limitation of this study is the use of patient recollection of Lyme disease diagnosis by a physician, and personal reporting of subjective symptoms by the respondents. Because the survey was random and anonymous, follow up with respondents or their physicians regarding the criteria used to diagnose their disease was not possible. However, the patient perspective is a neglected area of concern[31] and one that is fraught with controversy, as described in the report issued by the Institute of Medicine (National Academy of Science) titled “Critical Needs and Gaps in Understanding Prevention, Amelioration, and Resolution of Lyme and Other Tick-Borne Diseases”[32]. The IOM report on Lyme disease concluded that there is a need for greater understanding of the burden of illness faced by people with the disease, their families and society.
5. Conclusions
- The current CSTE case definition, which is the basis for the both CDC reporting criteria and guidelines for physician diagnosis of Lyme disease, are weighted heavily toward reliance on what are widely considered to be objective clinical signs of infection by B. burgdorferi: an erythema migrans rash, which occurs in the early stages of infection and predominantly when the patient is bitten by a nymph stage tick, and a positive serological test with poor reported sensitivity. These criteria may be exclusive of a diagnosis of Lyme disease in a patient with early stage or disseminated (late-stage) Lyme disease, one whose disease was transmitted by an adult tick, or one infected with other or multiple tick-borne pathogens. This in turn leads to underreporting of Lyme disease cases to local, state, and national public health systems and a concomitant underestimation of Lyme disease risk. A more reliable system of case reporting is needed to enable public health officials to better estimate the true burden of Lyme disease and identify areas where resources and education are need for improved public health.This study highlights the disparity that exists between Lyme disease reporting and the actual prevalence of the disease as determined by the number of people who reported being diagnosed with Lyme disease and those with symptoms consistent with Lyme disease who had not been diagnosed. This may be attributed at least in part to the existence of two evidence-based, nationally published sets of diagnostic and treatment guidelines for Lyme disease[33, 34], which creates confusion and misconception on the part of physicians with regard to the current diagnostic criteria, in addition to a hostile medicolegal environment for physicians who diagnose or treat patients outside of the guidelines[35]. The current CDC recommendations for diagnosis and treatment of Lyme disease do not specifically address nor provide options for people with undetected disease that fail to meet the current clinical or serological criteria. However, this study demonstrates that the size of this population may be quite large, with needs that are currently unmet at all levels of public health.
ACKNOWLEDGEMENTS
- Kind thanks to student research assistants Daniel Moynihan and Carla Steves, who disseminated and collected the surveys used in this study. Also SUNY Adirondack Director of Institutional Research David Smith, who scanned and converted the survey data to an electronic format.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML