-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Microbiology Research
p-ISSN: 2166-5885 e-ISSN: 2166-5931
2013; 3(6): 255-260
doi:10.5923/j.microbiology.20130306.10
Bioassay-directed Isolation and Evaluation of Harmine from the Terrestrial Plant Peganum harmala L. for Antibacterial Activity against Flavobacterium columnare
Kevin K. Schrader1, Charles L. Cantrell1, Leonid K. Mamonov2, Tatyana S. Kustova2
1United States Department of Agriculture, Agricultural Research Service, Natural Products Utilization Research Unit, National Center for Natural Products Research, University, Mississippi 38677-8048, United States of America
2Institute of Plant Biology and Biotechnology, Timiriazeva 45, Almaty 05040, Republic of Kazakhstan
Correspondence to: Kevin K. Schrader, United States Department of Agriculture, Agricultural Research Service, Natural Products Utilization Research Unit, National Center for Natural Products Research, University, Mississippi 38677-8048, United States of America.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Enteric septicemia of catfish (ESC) and columnaris disease in pond-raised channel catfish (Ictalurus punctatus) are caused by Edwardsiella ictaluri and Flavobacterium columnare, respectively, while Streptococcus iniae can cause streptococcosis in freshwater fish. During our search for natural compounds to replace antibiotics used to manage these diseases, the antibacterial activities of crude extracts obtained from the aerial portions and roots of Peganum harmala L. were evaluated against these common fish pathogenic bacteria species using a rapid bioassay. The ethanol extract of the roots of P. harmala was found to be the most active against F. columnare, with a 50% inhibition concentration (IC50) of 16 mg/L and a minimum inhibition concentration (MIC) of 10.0 mg/L. Bioassay-guided fractionation of this extract resulted in the isolation of harmine (7-methoxy-1-methyl-9H-pyrido[3,4-b] indole) as the active compound, with an IC50 of 3.8 mg/L and MIC of 10.0 mg/L. Harmine appears to be promising for evaluation of toxicity towards channel catfish and for efficacy studies to determine its potential use in managing columnaris disease in pond-raised channel catfish.
Keywords: Antibacterial, Aquaculture, Bioassay-guided Fractionation, Columnaris Disease, Flavobacterium columnare, Harmine, Peganum harmala
Cite this paper: Kevin K. Schrader, Charles L. Cantrell, Leonid K. Mamonov, Tatyana S. Kustova, Bioassay-directed Isolation and Evaluation of Harmine from the Terrestrial Plant Peganum harmala L. for Antibacterial Activity against Flavobacterium columnare, Journal of Microbiology Research, Vol. 3 No. 6, 2013, pp. 255-260. doi: 10.5923/j.microbiology.20130306.10.
Article Outline
1. Introduction
- The terrestrial plant Peganum harmala L. (Zygophyllaceae) is a perennial herb that is native to parts of southern Asia, the Middle East, and North Africa, with introduction and distribution also in parts of the mid-western United States[1]. The pharmacological activities of P. harmala have been widely studied for various human health benefits including antitumor[2], antinociceptive[3], and antibacterial[4]. Several studies have evaluated the antibacterial activities of the P. harmala seed extracts and extract constituents[5,6,7], and a more recent study examined the antibacterial activities of the different parts (e.g., leaf, flower, seed, and stem) of P. harmala against 13 different species of Gram-positive and Gram-negative human pathogenic bacteria[8]. However, no studies have examined the antibacterial activities of P. harmala extracts against common fish pathogenic bacteria.The most common bacterial diseases of pond-raised channel catfish (Ictalurus punctatus) in the southeastern United States are columnaris disease and enteric septicemia of catfish (ESC). Columnaris disease-related problems are common to the channel catfish aquaculture industry and can also occur world-wide in many other species of freshwater fish (e.g., rainbow trout, salmon, and tilapia) that result in heavy economic losses to these aquaculture industries[9,10]. The etiological agent for columnaris disease is the Gram-positive, motile rod-shaped (2-10 µm in length) bacterium Flavobacterium columnare[11]. Columnaris diseases can result in severe necrosis of gill tissue, skin ulceration from systemic infection, and high mortalities in fish. The etiological agent for ESC is the Gram-negative, rod-shaped (1-3 µm in length) bacterium Edwardsiella ictaluri[10]. The gross lesions of ESC in channel catfish include pale gills, small depigmented lesions and/or ulcers (1-3 mm) on the backs of infected fish, open lesions along the central skull line between the eyes, and hemorrhage at the base of the fins, under the jaw, and on the belly. In addition to high mortality rates of pond-raised channel catfish, prevention and treatment approaches can cost producers millions of dollars annually[10].Another significant bacterial disease in farmed freshwater fish, though less so in pond-raised channel catfish, is streptococcosis which is a prevalent problem in fish species such as tilapias (Oreochromis spp.) and hybrid striped bass[Morone chrysops female x Morone saxatilis male (Percichthyidae)][12]. One streptococcal species of bacteria that has been attributed as the cause streptococcosis in freshwater fish is Streptococcus iniae, a Gram-positive spherical-shaped cell (0.5-2.0 µm in diameter). The gross lesions of streptococcosis in tilapia include hemorrhage in the skin and at the base of fins, epidermal lesions and/or bloody ulcers, and opaque corneas[10]. Because streptococcosis can result in very high mortality rates, management approaches include prevention as well as treatment once the disease is determined to be present within a population of fish.There are several available management approaches for columnaris disease and ESC including the application of medicated feeds, live attenuated vaccines[13], and nonantibiotic therapeutants such as 35% Perox-Aid® for external columnaris[10]. Perox-Aid® is not recommended for use in earthen ponds unless water can be exchanged in pond. Additional inorganic agents such as copper sulfate pentahydrate (CuSO4•5H2O) and potassium permanganate (KMnO4) have been discussed as potential treatments for columnaris disease[14], but the efficacy of these compounds can be adversely impacted by certain water quality variables, and extra care must be taken when utilizing these therapeutants due to their broad-spectrum toxicity towards non-target organisms (e.g., channel catfish)[15].Currently, only florfenicol (Aquaflor®) is approved in the United States of America (USA) for the treatment of streptococcal septicemia caused by S. iniae in warmwater fish. Preventive management approaches for streptococcosis should include maintaining high water quality, application of high-quality diets, adequate water exchange and disinfection, and adequate removal of fecal waste from recirculating water systems[10]. Vaccination also appears to be a very promising approach for protection against S. iniae infection in Nile tilapia[16].The discovery of novel, environmentally safe, natural antibacterial compounds would greatly benefit aquaculturists due to the limitations or the absence of current management approaches available for controlling the bacterial species responsible for columnaris disease, ESC, and streptococcosis. As part of our discovery process to identify active compounds against isolates of F. columnare, E. ictaluri, and S. iniae, crude extracts from various plants collected in Kazakhstan have been evaluated for evaluation using a rapid bioassay. During the evaluation of some of the crude extracts of plants from Kazakhstan, an active crude extract from the terrestrial plant Peganum harmala L. (Zygophyllaceae) was found to be promising and was subsequently chosen for bioassay-guided investigation studies. Bioassay evaluation of an isolated compound from a fraction of the crude extract was also performed.
2. Materials and Methods
2.1. Collection of Plant Materials
- The terrestrial plant Peganum harmala L. (Zygophyllaceae) was collected for evaluation. The aerial parts and roots of P. harmala were collected on August, 6, 2011, while flowering in Almaty, Kazakhstan at location coordinates: N=44014' 275'', E= 0750 46' 322''. A voucher specimen number 3958/18 has been deposited in the Institute of Botany and Phytointroduction herbarium, Almaty, Kazakhstan.
2.2. Crude Plant Extraction
- Air-dried aerial parts (160 g) were extracted at room temperature using 1.9 L of methylene chloride (CH2Cl2) providing 5.5 g of extractables after evaporation of solvent. Dried marc was subsequently extracted using 1.7 L of 95% ethanol providing 15.2 g of extractables following evaporation of solvents. This process was again repeated for the air-dried roots (200 g) providing 4.9 g of CH2Cl2 extractables and 9.3 g of 95 % ethanol extractables.
2.3. Analytical Instrumentation
- 1H and 13C NMR spectra were recorded in DMSO-d6 on a Varian ANOVA 400 MHz spectrometer (Varian, Inc., Palo Alto, California, USA). Column chromatography was performed using a Biotage, Inc. Isolera pump (Charlottesville, Virginia, USA) equipped with a Horizon flash collector and a dual-wavelength (254 and 280 nm) detector. Electron impact mass spectrometry (EI-MS) spectra were recorded on a Varian CP-3800 gas chromatograph coupled to a Varian Saturn 2000 mass spectrometer. Column chromatography was performed using a Biotage Isolera One flash purification system (Biotage, Uppsala, Sweden).
2.4. Bioassay-guided Isolation
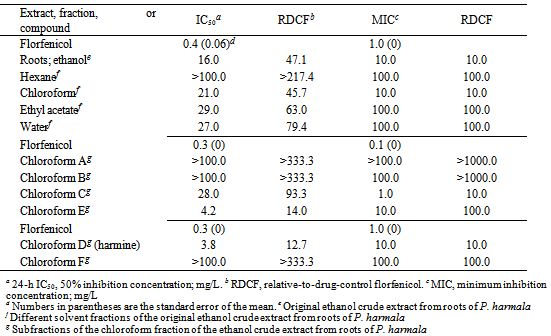
- Roughly 4.2 g of P. harmala ethanol extract from the roots was dissolved in 200 mL MeOH/H2O (90:10, v/v) and subjected to liquid/liquid partitioning with 150 mL of hexane (x3), obtaining 0.437 g of hexane extractables. To the remaining MeOH/H2O partition, 57 mL of H2O was added to make it 70:30 (v/v) MeOH/H2O. It was then partitioned with 150 mL chloroform (x3), obtaining 8.394 g of chloroform extractables. The remaining MeOH was removed by rotary evaporation, followed by the addition of 100 mL of ethyl acetate, obtaining 2.284 g of ethyl acetate extractables. The water extract was rotary evaporated to remove the residual organics, and the water was lyophilized, obtaining 1.5 g of water extractables. The chloroform partition was separated on the Biotage XP-Sil, 100g SNAP cartridge (40-63µm, 60Å, 39 x 157 mm) running at 40 mL/min using a hexane/isopropyl alcohol (IPA) gradient beginning with a linear step gradient from 100% hexane to 100% IPA over 1599 mL followed by 100 % IPA over 201 mL, and finishing with a MeOH wash of 350 mL. Collection was monitored at 254 nm and 220 nm. Portions of 22 mL each were collected into 16 x 150 mm test tubes. All test tubes were evaluated by thin-layer chromatography (TLC) with hexanes/IPA (1:1) on Anal Tech Silica Gel GF 250 µm plates. Ten test tubes (38-47) were combined and concentrated on the basis of TLC similarities, providing 0.396 g of crystalline harmine.
2.5. Harmine
- EI-MS m/z 213.1[M + H]+.1H NMR (400 MHz) δ 11.66 br s, 8.17 d, 8.07 d, 7.87 d, 7.03 s, 6.86 d, 3.87 s, 2.75 s. 13C NMR (101 MHz) δ 160.4, 142.4, 140.9, 134.5, 127.8, 122.9, 114.7, 112.2, 109.5, 94.6, 55.4, 19.9[20].
2.6. Test Bacteria and Standardization
- An isolate of E. ictaluri (isolate S02-1039) was obtained from Mr. Tim Santucci (College of Veterinary Medicine, Mississippi State University, Stoneville, Mississippi, USA). Cultures of E. ictaluri were maintained on 3.8% Mueller-Hinton (MH) agar plates (pH 7.3) (Becton, Dickinson and Company, Sparks, Maryland, USA) in order to assure purity. Prior to conducting the bioassay, single colonies of E. ictaluri were used to prepare the assay culture material by aseptically transferring bacterial cells from colonies to 45 mL of 3.8% MH broth to form a bacterial cell density of 0.5 McFarland standard[17].An isolate of F. columnare[isolate ALM-00-173 (genomovar II)] was obtained from Dr. Covadonga Arias (Department of Fisheries and Allied Aquacultures, Auburn University, Auburn, Alabama, USA). The purity of cultures of the F. columnare isolate was assured by streaking the bacteria for isolation on modified Shieh (MS) agar plates (pH 7.2-7.4)[18] and then verifying after incubation at 29±1℃ for 3-5 days that only one bacterial colony type was present. Prior to conducting the bioassay, single colonies of F. columnare were used to prepare assay culture material by culturing in 75 mL of MS broth (24 h) at 29±1°C at 150 rpm on a rotary shaker. After overnight incubation, a 0.5 McFarland standard of F. columnare assay material was prepared by aseptically transferring cells from the broth culture to fresh MS broth.A culture of S. iniae (isolate LA94-426) was provided by Dr. Ahmed Darwish (formerly at the U.S. Department of Agriculture, Agricultural Research Service, Harry K. Dupree Stuttgart National Aquaculture Research Center, Stuttgart, Arkansas, USA). The purity of cultures of S. iniae was verified by streaking the bacteria for isolation onto Columbia CNA agar plates containing 5% sheep blood (Remel, Inc., Lenexa, Kansas, USA) and then checking after incubation at 29±1℃ for 3-5 days that only one bacterial colony type was present. Bioassay culture material of S. iniae was prepared in the same manner as used for the F. columnare isolate, except 3.8% MH broth was used and the broth cultures were incubated for 18 h instead of 24 h prior to preparing the 0.5 MacFarland standard.
2.7. Test Bacteria and Standardization
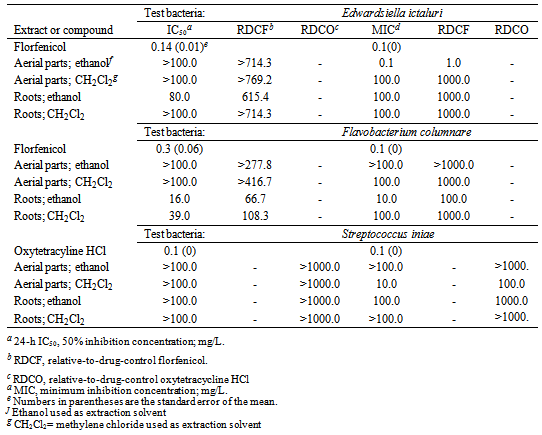
- The crude extracts from P. harmala, extract fractions, and any isolated pure compounds were evaluated for antibacterial activity using a rapid 96-well microplate bioassay and following the procedures previously reported [17]. Florfenicol and oxytetracycline HCl, antibiotics that can be included in medicated feed, were used as positive drug controls. Also, control wells (no test material added) were utilized in each assay. The initial crude extracts samples were dissolved in either CH2Cl2 or ethanol. Extract fractions obtained later were dissolved in either methanol or CH2Cl2 (also used for the initial chloroform, ethyl acetate, and hexane fractions) depending on the eluant used to obtain the extract fraction. Drug controls were dissolved in ethanol. Technical grade solvents were used in this study. Final test concentrations of the crude extracts and extract fractions in the microplate wells were 0.1, 1.0, 10.0, and 100.0 mg/L. Final concentrations of drug controls were 0.01, 0.1, 1.0, 10.0, and 100.0 µM. Three replications were used for each dilution of each crude extract, extract fraction, test compound, and controls. Final results of drug controls were converted to units of mg/L to allow comparisons with the other data.In order to determine the 24-h 50% inhibition concentration (IC50) and minimum inhibition concentration (MIC), sterile 96-well polystyrene microplates (Corning Inc., Corning, New York, USA) with flat-bottom wells were used for extracts and extract fractions dissolved in either ethanol or methanol while sterile quartz 96-well microplates (Hellma Cells, Inc., Forest Hills, New York, USA) were used for extracts, extract fractions, and isolated test compounds dissolved in CH2Cl2. Initially, dissolved test material or drug controls were micropippeted separately into individual microplate wells (10 µL/well), and solvent was allowed to completely evaporate before 0.5 MacFarland bacterial culture was added as described previously[17] to the microplate wells (200 µL/well). Microplates were incubated at 29±1°C. A SpectraCount microplate photometer (Packard Instrument Company, Meriden, Connecticut, USA) was used to measure the absorbance (630 nm) of the microplate wells at time 0 and 24 h.The means and standard deviations of absorbance measurements were calculated, graphed, and compared to controls to help determine the 24-h IC50 and MIC for each crude extract, extract fraction, and isolated test compounds [17]. The 24-h IC50 and MIC results for each test fraction and compound were divided by the respective 24-h IC50 and MIC results obtained for the positive controls florfenicol (for E. ictaluri and F. columnare) and oxytetracycline (for S. iniae) to determine the relative-to-drug-control florfenicol (RDCF) and relative-to-drug-control oxytetracycline (RDCO) values.
3. Results
- Among the different crude extracts that were evaluated, the ethanol extract of the aerial parts of P. harmala showed the strongest activity against E. ictaluri based on MIC results, with a MIC of 0.1 mg/L (Table 1). However, based on IC50 results, there was no activity (IC50 >100.0 mg/L) against E. ictaluri at the test concentrations that were evaluated. The other three crude extracts were either not active or showed low activity against E. ictaluri at the test concentration used in the study. The most active crude extract against F. columnare was the ethanol extract of the roots of P. harmala, with an IC50 of 16.0 mg/L and a MIC of 10.0 mg/L (Table 1). The CH2Cl2 extract of the roots was also active against F. columnare, with an IC50 of 39.0 mg/L; however, the MIC of 100.0 mg/L was higher than the ethanol extract of the roots. The other two crude extracts showed little, if any, activities against E. ictaluri at the test concentrations used in the study.Based on MIC results, the most active crude extract against S. iniae was the CH2Cl2 extract of the aerial parts of P. harmala, with a MIC of 10.0 mg/L (Table 1). However, there was no observed activity of this same extract against S. iniae based on IC50 results (IC50 >100.0) and at the test concentrations utilized in this study. The other three extracts showed little or no activities against S. iniae based on IC50 and MIC results. Overall, the activity of the ethanol extract of the roots of P. harmala against F. columnare appeared to be the most promising among the four different crude extracts and when considering the three test bacteria used in this study. Subsequently, bioassay-guided fractionation of this extract was performed.
|
|
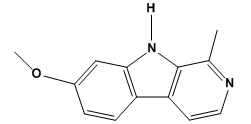 | Figure 1. Chemical structure of harmine (7-methoxy-1-methyl-9H-pyrido [3, 4-b] indole) |
4. Discussion
- Our study is the first to examine the crude extracts from different parts of P. harmala for activities against several common species of fish pathogenic bacteria (E. ictaluri, F. columnare, and S. iniae). The initial evaluation of the crude extracts revealed significant activity by the roots extract against F. columnare. Therefore, this extract was chosen for bioassay-guided fractionation in order to isolate the active compound (s). Additional separation of the most active chloroform partition revealed moderate to strong activities of two of the sub-fractions (chloroform D and E). Sub-fraction chloroform D had slightly stronger activity against F. columnare than sub-fraction chloroform E, and, therefore, the focus was then to identify the active compound(s) in sub-fraction chloroform D. Using 1H and 13C NMR spectroscopy, sub-fraction chloroform D was determined to be comprised of only one compound that was identified to be harmine by comparison of NMR data with that reported in the literature[20].Harmine has previously been identified as possessing antibacterial activities against several species of bacteria. Shahverdi et al.[5] reported on the significant activity of the smoke preparation of a dichloromethane extract of seeds of P. harmala against several species of Gram-positive bacteria including Bacillus subtilis, Staphylococcus aureus, and Staphylococcus epidermidis. They determined that harmine was the most prevalent compound in this extract, and evaluation of standard harmine against nine Gram-postive and Gram-negative test bacteria revealed activity against eight of the test bacteria. Nenaah[7] also reported on the antibacterial of harmine against Bacillus subtilis and harmine was found to also be very effective against the Gram-negative bacterium Proteus vulgaris. An efficacy study by Arshad et al.[19] evaluated the effects of the incorporation of P. harmala seed extracts in feed for poultry in order to minimize infections by the Gram-negative bacterium Escherichia coli. Their results indicated limited antimicrobial activity of the P. harmala seed extracts against E. coli in vivo, and they concluded that long-term continuous feeding for six weeks might also lead to undesired side effects in the chickens (e.g., adverse impact on liver function). However, in their study, they did not evaluate the pure compound harmine or any other compounds present in the test crude extracts. Prior to any efficacy challenge studies of harmine for columnaris disease in channel catfish, long-term feeding studies should be performed to assess the potential toxicity of harmine towards catfish. Challenge studies could also evaluate the use of harmine with florfenicol, so that reduced doses of florfenicol would be required in order to effectively manage columnaris disease in channel catfish. In addition, analogs of harmine should also be evaluated in vitro to determine if greater activity against F. columnare can be achieved.
5. Conclusions
- The results of this study demonstrate the in vitro antibacterial activity of the dichloromethane extract obtained from the roots of P. harmala against F. columnare. Bioassay-guided fractionation of this dichloromethane extract identified harmine to be the main active compound present, with moderate to strong activity against F. columnare. Harmine is promising for evaluation of toxicity towards channel catfish and for efficacy studies to determine its potential in managing columnaris disease in pond-raised channel catfish.
ACKNOWLEDGEMENTS
- The authors thank Ms. Phaedra Page, Mr. Solomon Green III, Mr. Marcuslene Harries, and Ms. Amber Reichley, Natural Products Utilization Research Unit, USDA-ARS, for technical assistance. Financial support in part from the International Science and Technology Center (ISTC) project K1896 is greatly appreciated.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML