-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Microbiology Research
p-ISSN: 2166-5885 e-ISSN: 2166-5931
2013; 3(6): 247-254
doi:10.5923/j.microbiology.20130306.09
Antituberculosis Activity of Bioactive Compounds from Fruit Extract of Acacia nilotica
Oladosu P. O.1, Isu N. R.2, Ibrahim K.1, Orishadipe A. T.3, Lawson L.4
1National Institute for Pharmaceutical Research and Development, Idu Industrial Area, Garki, Abuja, Nigeria
2University of Abuja, Department of Biological Sciences, Gwagwalada Abuja, Nigeria
3Chemistry Advanced Laboratory, Sheda Science and Technology Complex, Abuja, Nigeria
4Zankli Medical Centre, Tuberculosis Research Laboratory, Mabushi, Abuja, Nigeria
Correspondence to: Oladosu P. O., National Institute for Pharmaceutical Research and Development, Idu Industrial Area, Garki, Abuja, Nigeria.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Three antituberculosisbio active compoundsviz garlic acid, methyl garlic and catechinwere isolated from fruit extract of Acacia nilotica by high throughput chromatography techniques – column chromatography, high performance liquid chromatography and Liquid chromatography – Mass spectra and characterized by Nuclear magnetic resonance. The isolated compounds tested against Mycobacterium tuberculosis by broth microdilution method were significantly active at 62.5, 31.25 and 20 µg/µl respectively. The implication of these results is that the isolated compounds can serve as lead in the development of antituberculosis drugs.
Keywords: Antituberculosis, Acacia Nilotica, Bioactive Compounds, Garlic Acid, Methyl Gallate, Catechin
Cite this paper: Oladosu P. O., Isu N. R., Ibrahim K., Orishadipe A. T., Lawson L., Antituberculosis Activity of Bioactive Compounds from Fruit Extract of Acacia nilotica, Journal of Microbiology Research, Vol. 3 No. 6, 2013, pp. 247-254. doi: 10.5923/j.microbiology.20130306.09.
Article Outline
1. Introduction
- Tuberculosis continues to be a global concern and leading cause of deathattributable to a single infectious organism[9] and one third of the world’s population is infected with the pathogen[4]. According to WHO report[19], Nigeria ranked 4th among 22 high burden countries of tuberculosis based on estimated number of incidence cases.The current arsenal of front line therapeutics was developed half a century ago. Many of the individual antimycobacterial agents have low potency, and resistance to them is increasingly common, thus, there is need for the development of new Tb drugs, to shorten the duration of tuberculosis chemotherapy with little or no toxicity.Tuberculosis was almost eradicated but it resurfaced as a result of many factors: poverty and population increase, overcrowding, emergence of HIV/AIDS infectionand multi drug resistant tuberculosis.It is a re-emerging disease as a result of emergence of HIV/AIDS infection. The emergence of multi-drug resistant and extremely drug resistant strains of tuberculosis has necessitated the need for research into new drug for the treatment of tuberculosis/.One of the leading sources for the discovery of new drugs is medicinal plants. Acacia nilotica is one of the antituberculosis plants documented through a survey of medicinal plants used traditionally in the treatment of tuberculosis in some parts of Northern Nigeria[15]. It is an imperative multipurpose plant that has been used broadly for the treatment of various diseases[3]. It has also been reported to have antibacterial activity against Streptococcus viridans,Staphylococcus aureus, Escherichia coli, Bacillus subtilisand Shigelliasonnei[18]. There are other reports stating its uses for stomach upset and pain, against scurvy and the infusion is taken for dysentery and diarrhoea. Literature is however is scarce in respect of the efficacy of Acacia nilotica extract as antituberculosis agent. The antituberculosis activity of the isolated compounds from A. nilotica is therefore being reported for the first time by this study.Thus, A. nilotica looks promising as a potential lead in drug development from herbal medicine for the treatment of tuberculosis.
2. Methodology
2.1. Plant Collection and Identification
- The plant was collected in Suleja, Niger state, Nigeria through ethnobotanical survey using a modified questionnaire. The plant was identified by aEthnobotanist at the National Institute for Pharmaceutical Research and Development, Abuja, Nigeria.
2.1.1. Plant Extraction
- The fruit pulp of the plant was air dried at room temperature (28℃) to constant weight and pulverized using a crushing machine (Trapp Metallurgical, Trapp Ltd, Brazil). Ten gram of pulverized plant part was soaked in 100 ml 70% aqueous methanol for 24 hours to extract both polar and non-polar components of the plant. The exudate was filtered and concentrated on a rotary evaporator at 40℃ (Fig. 1).
2.2. Bioassay Guided Fractionation
- This was carried out according toOrishadipe[16]. Activated silica gel (50 g) was packed by wet packing method into a column (Fig. 2). The extract (2.6 g) which was absorbed on silica gel (60-120 mesh) and dried was loaded on the column. Gradient elution was performed with 100 ml of each mobile phase mixture in the column. Gradient elution was performed with 100 ml of each mobile phase mixture in a series. The elution was performed in flash chromatography as described in[16]. The mobile phase consisted of hexane, ethylacetate, methanol and water with 100% hexane and 10% increment in the next polar component. The final elution was performed with 70% methanol in water until the column appeared exhausted with a sign of colourless silica gel. The eluates were monitored by thin layer chromatography using normal phase pre-coated silica gel KC5 TLC plates. The TLC mobile phase consists of a mixture of ethylacetate: hexane (7:3). The eluates were combined based on the similarity of TLC fingerprint to give six fractions labelled as FR1-FR6. The six fractions were screened separately for antituberculosis activity. Fractions 2, 3 and 4 had similar antituberculosis results and TLC finger printing similarity, therefore were pulled together and purified further to isolate the respective compounds. About 3.8 g of the active fraction was absorbed on Merck – Kiesegel and introduced into column packed with the same adsorbent. It was eluted with petroleum ether and an increasing gradient of ethyl acetate. A total of 6 fractions of 100 ml each were collected. The fractions with similar TLC similarities were pooled together into 3 portions, labelled as portions 1-3, and purified further by HPLC technique. This was done by separating them on a reverse phase column, eluting with a gradient of acetonitrile 5% - 98% in water for a period of 40 minutes at 2 nM wave length. Some 100 mg of extract was dissolved in1ml methanol, water was added gradually to the point of precipitation before injecting on the HPLC reversed phase column. Fractions were collected at intervals of one minute. A total of 60 fractions were collected, concentrated on rotary evaporator and lyophilized on freeze drier. Only fraction 3 to 16 contained some compounds as indicated by nuclear magnetic resonance (NMR) coupled to the HPLC. The fractions were analysed on Liquid Chromatography-Mass Spectrometer (LCMS) for purity and also mass. From the aforementioned analyses, fractions 1-3 through a digital detector in the HPLC as described in (6) was found to contain three compounds which were isolated by preparative HPLC separation on a reversed phase (C-18) column. The pure sample compounds were fractions 5, 9 and 12. Fractions in between were mixtures of these compounds as indicated by the NMR spectra. These compounds, F5, 9 and 12 were characterized and elucidated using NMR coupled with LC-MS and confirmed with standards.
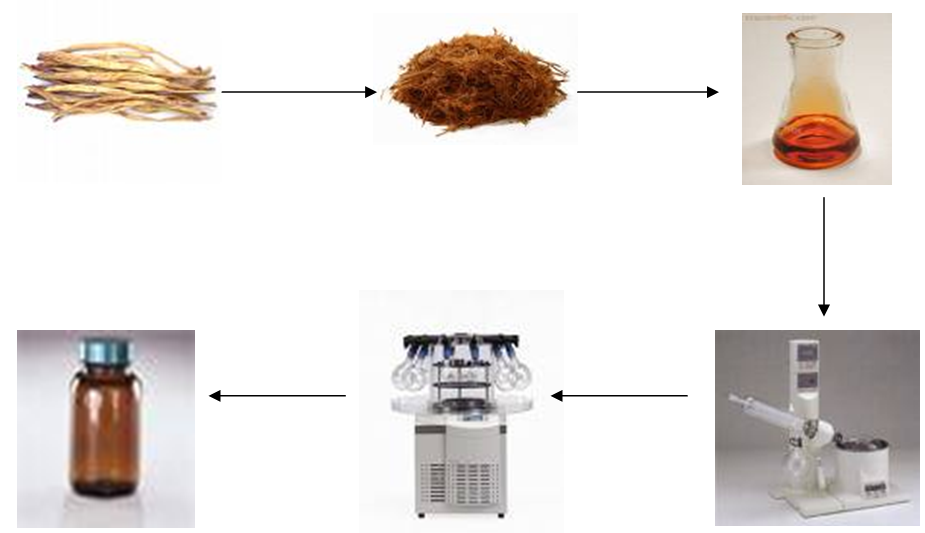 | Figure 1. Schematic chart of extraction process of A. nilotica |
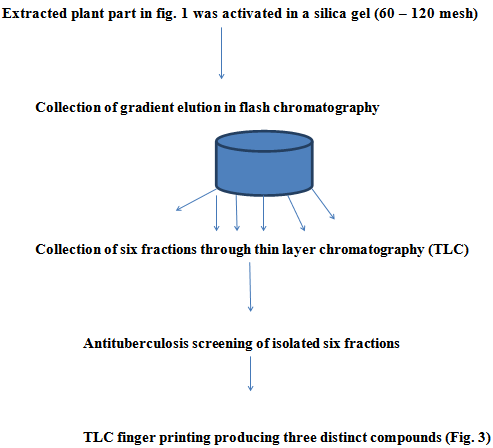 | Figure 2. Flow chart of fractional isolation procedure of A. nilotica |
 | Figure 3. Thin Layer Chromatography plate of three distinct compounds |
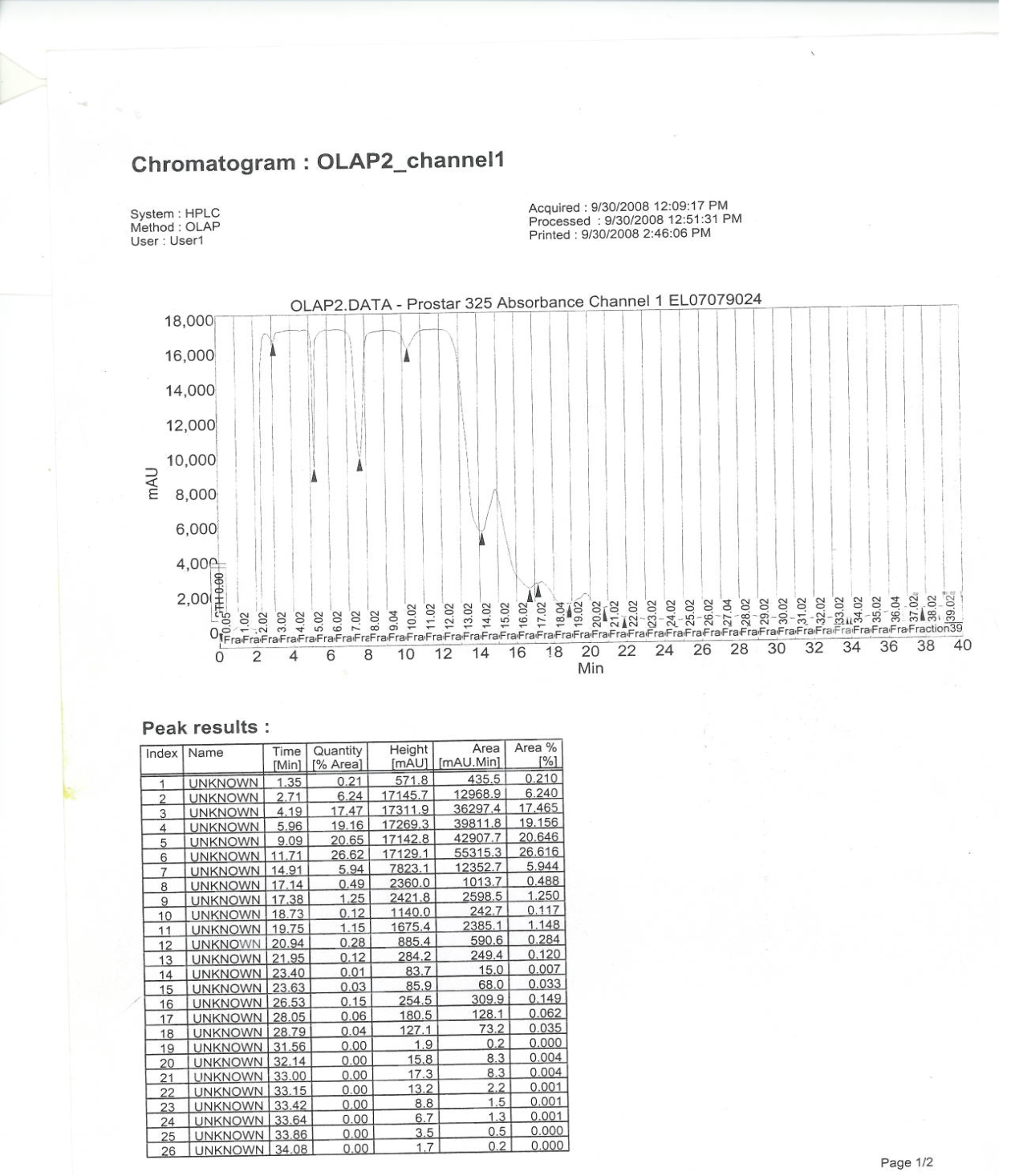 | Figure 4. HPLC NMR Spectra of the three isolated compounds |
2.2.1. Structural Elucidation of Isolated Compounds
- The structural elucidation of the compounds was done using NMR (300MHZ Varian machine) and LC-MS (Agilent) techniquesas described by Pourchet et al.,[17] andOrishadipe[15]. TLC fractions were dissolved in deuterated methanol (CD3OD) and transferred to NMR tube. The tube was later transferred to the probe for NMR measurements of proton (1H) and 13C (carbon 13). Mass were acquired using an LC-MS.
2.3. Antimycobacteria Determination of the Isolated Compounds and Crude Extract of the Plant
- The isolated compounds identified asgallic acid, methyl gallate,catechinand the crude had the following yields 10, 20, 30 and 100 mg respectively and were dissolved in 0.5 ml DMSO except the crude that was dissolved in 1 ml DMSO. The solutions were further diluted in the ratio of 1:10 by adding 50 µl of compound to 450 µl of7H9 Middlebrook supplemented with albumin dextrose (ADC) broth to give the following concentrations; 2, 4, 6 and 10 mg/ml respectively. The samples were screened for antimycobacterial activity in sterile 96 micro well plates by broth microdilution methods according to Barry[4]. Into each of the wells of 96 micro well plate was transferred 50 µl of sterile 7H9 broth starting from well 2 to 12. To each of the first wells was added 100 µl of 10% DMSO in sterile media (prepared by dispensing 0.1 ml of DMSO into 9.9 ml of 7H9 broth as control), 100 µl of 25 µg/ml solution of isoniazid (prepared by dissolving 250 mg of isoniazid powder in 10 ml DMSO and diluted in 1: 1000 by dispensing 25 µl of isoniazid in 25 ml 7H9 Middlebrook/ADC broth) and 100 µl of crude plant extract. Using a multi-channel pipettor, 50 µl was carefully removed from well 1 and added to well 2, mixed thoroughly by pipetting up and down four times, and the process continued to well 11 from which 50 µl was withdrawn and discarded.
2.3.1. Resuscitation of Test Organisms
- Five hundred microliter of test organisms (M. bovis (BCG) freshly thawed stock was inoculated into 50 ml of sterile Middlebrook 7H9/ADC broth media from a frozen saturated stock solution in 50% glycerol and placed at 37℃ with shaking for 5-7 days. The optical density of the resulting culture was measured using a uv/spectrophotometer (6405 Jenway, Barloworld Scientific Ltd. Dunmow, Essex CMB 3LB). The resulting optical density (OD) of resulting culture determined at 650 nm was approximately 0.2) diluted 1/1000 by adding 50 µl cell culture to 50 ml 7H9/ADC medium, where 50 µl of diluted culture was inoculated to all wells of the plate. The plates were incubated at 30℃ for 5 – 7 days. After the incubation period the plates were observed for inhibition or growth of M. tb and M. bovis (BCG). Growth was identified by turbidity. MIC was determined as the last concentration where no growth was observed.
2.3.2. Synergistic Antimycobacterial Study of Isolated Compounds
- The synergistic effect of the isolated compounds against tuberculosis was carried out as reported by Nachimutuet al.,[13]. The HPLC sample containing the three compounds; methyl gallate, galic acid and catechin with a yield of (10.24 mg) was dissolved in 1 ml 7H9 broth and screened against M. bovis and M. tb. For the combination assay, 10 mg of each compound was dissolved in 1 ml DMSO and diluted in 7H9 broth by adding 50 µl of dissolved compound to 450 µl of medium (1:10). Fifty microliter of each of the three compounds was mixed together in twos in the following order;[1] gallic acid + methyl gallate,[2] gallic acid + catechin[3] methyl gallate + catechin. Some 100 µl of the mixtures was transferred to first well of a sterile microwell plate. From well 1, 50 µl of the mixture was transferred to well 2 containing 50 µl broth, mixed thoroughly using multi-channel pipette and the procedure was continued to well 11 where 50 µl was discarded to have equal volume (50 µl) all through. Compound dilutions were made to allow the inhibitory concentration to fall at about the fourth two fold serial dilution. All the wells were inoculated with 50 µl of each of M. tb and M. bovis (BCG). The microwell plates were incubated 300 C for 5-7 days. After the incubation period, the plates were observed for growth or inhibition of test organisms using inverted macroscopic mirror.
3. Results
3.1. Characterization of Isolated Compounds
- The first compound was a white solid with a melting point of 202-204℃ (Litt 201-204℃). The 1HNMR revealed the presence of aromatic protons at δ7.05, which integrated for two protons and that of methoxyl at δ3.3. This indicated a simple aromatic system. The broad band was decoupled as; 1H NMR: 7.0 (2H, H-2 and H-6), 5.0 (OH), 3.90 (3H, CH3), 13C NMR: 167.9 (C-7), 146.6 (C-3), 146.6 (C-5), 139.4 (C-4), 122.2 (C-1), 110.3 (C-2), 110.3 (C-6), 52.5 (OMe).13CNMR revealed the presence of 8 carbons at δ (167.9 for 1 C=O, 110.3 for 2CH, 52.5 for OCH and 4 quaternary aromatic carbons at 122.2, 138.4, 146.6, 146.6). HRMS of this fraction gave 184.03717 amu, which was consistent with molecular formula C8H805. A library search revealed it to be methyl gallate[14] and[16]. The sample was also comparedwith an authentic sample by TLC and was confirmed to be methyl gallate.(Fig 5).
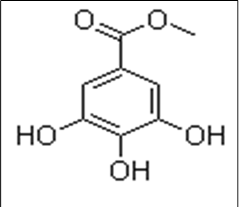 | Figure 5. Chemical structure of Methyl gallate |
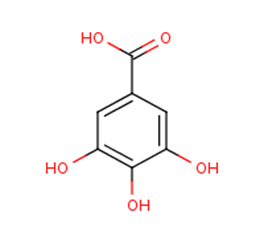 | Figure 6. Chemical structure of gallic acid |
 | Figure 7. Structure of catechin |
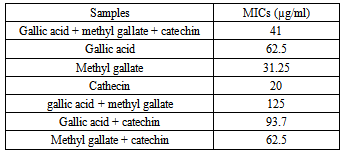
3.2. MIC of Antimycobacterial Study of Methyl Gallate, Gallic Acid and Catechin
- From the data, the mics of the three compounds were observed to be varied. MIC value of cathecin (20.0) is significantly active (p<0.05) than the mic value of methyl gallate (31.25) and gallic acid (62.5) µg/ml respectively. However, the MICs of the three compounds combined are significantly active than the micof the crude extract 625 µg/ml (Fig. 8).
3.3. Synergistic Antimycobacterial Study of Isolated Compounds
|
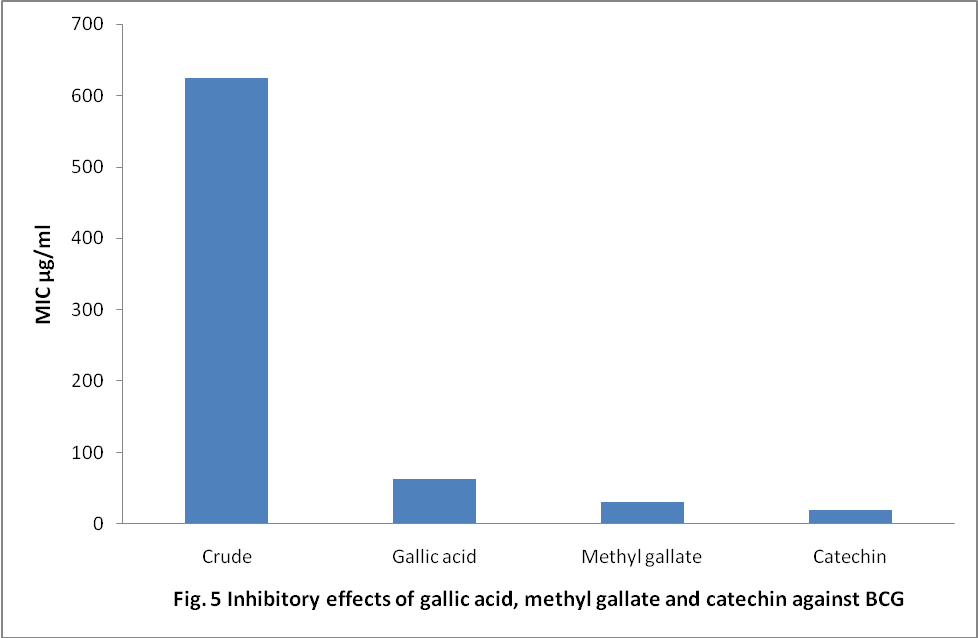 | Figure 8. inhibitory effects of gallic acid, methyl gallate and catechin against BCG |
4. Discussion
- Purification of the fractions by HPLC technique led to isolation of three distinct compounds and were analysed by NMR and LC-MS for compounds identification. The three compounds were identified as gallic acid, methylgallate and catechin. Methyl gallate and gallic acid have been reported to have antituberculosis activities by Orishadipe[15] from Endrophragmaangolensis which agrees with the findings in this study, that mic of gallic acid (62.5 µg/ml) from E. angolensis was same as that of A. nilotica but not as significantly (p>0.05) effective as catechin[20] and methyl gallate (31.25 µg/ml) respectively. The significant effect (p<0.05) of methyl gallate (31.25) as compared to gallic acid (62.5 µg/ml) could be due to the presence of the methyl group on the methyl gallate structure which confers lypophilic tendency on the molecule compared to the carboxylic acid of the gallic acid which is highly hydrophilic. The lypophilic tendency of the methyl group on methyl gallate gives it ability to bind to the cell membrane of M. tuberculosis probably through the lipid pathway. This result agrees with the report of Mativandlela[11], that antituberculosis activity of flavanoids from Galeniaafricana inhibited M. tuberculosis at 50 µg/ml, anmic close to the findings in this work. In this study, catechin at (20 µg/ml) is the most significantly (p<0.05) active as compared to the other 2 compounds gallic acid and methyl gallate). The name of the catechin chemical family derives from catechu according to Zhenget al., (21) is the juice or boiled extract of Mimosa catechu (Acacia catechu L. f). It possesses two benzene rings (called the A- and B-rings) and a dihydropyranheterocycle (the C-ring) with an hydroxyl group on carbon 3 (Fig 3). The A ring is similar to a resorcinol moiety while the B ring is similar to a catechol moiety. There are two chiral centers on the molecule on carbons 2 and 3. It has therefore four diastereo isomers. Two of the isomers are in trans configuration and are called catechin and the other two are in cis configuration and are called epicatechin. It has been reported by different authors to have different biological activities. Cecile et al.,[6] reported that, catechin has an antioxidant activity, it has been found to be the most powerful scavenger between different members of the different classes of flavonoids. The ability to quench singlet oxygen seems to be in relation with the chemical structure of catechin, with the presence of the catechol moiety on ring B and the presence of a hydroxyl group activating the double bond on ring C[6]. Transcriptomic studies according to Sylvinaa et al.,[17] shows that catechin reduces atherosclerotic lesion development in apo E-deficient mice. (+)-Catechin inhibits intestinal tumor formation in mice (12). (+)-Catechin inhibits the oxidation of low density lipoprotein (9). (-)-Catechin suppresses expression of Kruppel-like factor 7[7]. Catechin shows an enhancement of the antifungal effect of amphotericin B against Candida albicans[10]. Incubation experiments with (+)-catechin show a prevention of human plasma oxidation[17].There was no report of antituberculosis properties of catechin found in the literature apart from other biological activities; hence it is being reported for the first time in this study. The significant antituberculosis of this compound could serve as a tractable system towards the development of antituberculosis drug. In conclusion, inhibition of the test organisms by these flavanoidsin vitro implied that they could be used as potential antituberculosis agents in drug development as other authors[1] have reported that flavanoid compounds are known to exhibit antibacterial properties.
ACKNOWLEDGEMENTS
- 1. This study was sponsored through a 2008 Ph.D grant by Nigerian Conservation Foundation in memory of Chief S. L. Edu, Km 19, Lagos - Express way, Lekki Lagos State, Nigeria. We remain grateful for this support.2. The Authors are grateful to the Management of Zankli Medical Centre, Mabushi, Abuja, Nigeria for the opportunity given to make use of the TB Research Laboratory during this study.3. The Authors wishes to acknowledge the support of the TB Research Lab of NIAID, National Institute of Health, Maryland, USA for the permission to make use of the TB Lab and NMR 300 MHZ Varian machine.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML