-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Microbiology Research
p-ISSN: 2166-5885 e-ISSN: 2166-5931
2013; 3(6): 228-233
doi:10.5923/j.microbiology.20130306.06
Human Papillomavirus (HPV) in Oral Cavity Lesions: Comparison with Other Oral Cancer Risk Factors
Raúl Fernando Venezuela 1, 2, Ángel Talavera 3, María Celia Frutos 1, Ana Ximena Kiguen 1, Marina Soledad Monetti 1, María Sollazo 3, René Panico 3, Ruth Ferreyra de Prato 3, Cecilia Gabriela Cuffini 1
1Institute of Virology, National University of Cordoba. Córdoba, 5016, Argentine
2Enfermera Gordillo Gómez s/n Ciudad Universitaria, Córdoba, 5016, Argentine
3Faculty of Dentistry - National University of Córdoba, 5016. Argentine
Correspondence to: Raúl Fernando Venezuela , Institute of Virology, National University of Cordoba. Córdoba, 5016, Argentine.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Human papillomavirus (HPV) is considered a necessary factor for the development of cervical cancer; however, its relationship with oral cancer is controversial. The aim of this study was detect the presence of HPV in lesions of the oral cavity and its correlation with other risk factors. Presence of HPV was studied by polymerase chain reaction in samples from benign lesions, potentially malignant lesions (PML), neoplasias and healthy mucosae. The results from the different groups were compared; in addition to their histopathological variables with tobacco smoking, alcohol consumption, so on. HPV was detected in 88.89% of benign lesions, 41.38% of PML and 56.25% of neoplasias. The most prevalent genotypes were 16 and 6. Together, reached 55% of the total number of cases. A significant association was observed between HPV and male gender, tobacco smokers, alcohol drinkers and benign lesions. Tobacco smoking and alcohol intake were associated to neoplasias. Our results showed that factors like tobacco smoking and alcohol drinking, have more influence than HPV in the development of oral neoplasias; however, 56.2% of the neoplasias tested positive for HPV; the percentage of HR-HPV detection increased with the severity of the lesions, suggesting its possible involvement in malignant processes
Keywords: HPV, Oral cavity lesions, Argentina
Cite this paper: Raúl Fernando Venezuela , Ángel Talavera , María Celia Frutos , Ana Ximena Kiguen , Marina Soledad Monetti , María Sollazo , René Panico , Ruth Ferreyra de Prato , Cecilia Gabriela Cuffini , Human Papillomavirus (HPV) in Oral Cavity Lesions: Comparison with Other Oral Cancer Risk Factors, Journal of Microbiology Research, Vol. 3 No. 6, 2013, pp. 228-233. doi: 10.5923/j.microbiology.20130306.06.
1. Introduction
- Head and neck cancer is a major health problem worldwide that usually appears in patients older than 50 years of age; however some studies have shown that between 1 to 6 percent of oral malignant tumors have been found in patients younger than 40[1,2,3].Head and neck cancer defines a heterogeneous group of malignant lesions that involve different sites with similar risk factors and pathological features. The majority of these malignancies are oral squamous cell carcinomas (OSCC) and are characterized by a multifactorial etiopathogenesis [4,5].The causal association between tobacco consumption and alcohol intake with the development of OSCC is well established; however, a considerable proportion of OSCC occurs in non smokers and non drinkers, indicating the presence of other risk factors[5,6,7].During the last decades, epidemiological and molecular data have indicated the involvement of high-risk human papillomavirus (HR-HPV) in these diseases, which was first proposed by Syrjanen in 1983 and afterwards supported by other authors[5,8,9,10] on the basis of the epitheliotropic nature of HPV[11,12], in addition to the the widely confirmed oncogenic potential of HR-HPV in the pathogenesis of anogenital cancer, especially cervical squamous cell carcinoma[13,14] and the morphological similarities between oropharyngeal and genital epithelia [15].Although HPV has been found in OSCC, the wide range of viral prevalence reported in the literature has not contributed to the clarification of the relationship between HPV and oral carcinogenesis[16,17].The aim of this study was to determine the frequency of HPV detection and circulating genotypes in lesions of the oral cavity as well as their correlation to other risk factors.
2. Material and Methods
- Patients: This case-control epidemiological study was performed in patients diagnosed and treated at the Faculty of Dentistry - National University of Córdoba (patients were included from March 2012 to December 2012 and control cases from October 2012 to December, 2012). All the patients underwent a careful analysis of their clinical records and signed a written informed consent before being included into the study.The patients presented histopathological diagnosis of benign lesions (vegetative lesions, wart and condylomateous) potentially malignant lesions (leukoplakia, lichen, verrucous leukoplakia, keratoacanthoma)[18] and neoplasias (verrucous carcinoma, carcinoma in situ-CIS and OSCC). Control cases were individuals with healthy oral mucosae who attended the Faculty of Dentistry for tooth extractions (Control group).The data were classified according gender, age (greater or younger than 50 years), conventional risk factors (smoking and drinking) site, histopathological diagnosis and cytological classification of the lesions.Consideration of an individual as a smoker and/or drinker was based on the study of Herrero 2003[19].The study population comprised 84 patients (9 with benign lesions, 29 potentially malignant lesions, 16 neoplasias and 30 control subjects).Sample collection: All participants were subjected to an oral examination for the collection of cells (brushing) for HPV-DNA detection. Patients with presence of lesion, post-brushing for HPV detection and cytology diagnosis (Papanicolau or PAP stains), underwent a biopsy which was collected in paraffin blocks. For control group was done brushing of full mouth.The brushing samples for PCR were collected in 500 μL phosphate-buffered saline solution (PBS). HPV detection: Viral DNA was extracted using the commercial AccuPrep Genomic DNA Extraction Kit (Bioneer Inc., CA, USA), in accordance with the manufacturer’s instructions.A 450-bp segment, corresponding to the L1 region of the viral genome, was amplified by PCR, using the degenerate consensus primers MY09 and MY11 (Integrated DNA Technology - USA)[20]. The product was detected by electrophoresis in 1.5% agarose gel using a U.V. transilluminator. The β-globin gene was used as a DNA preservation marker[21]. Negative samples were considered inadequate.HPV-DNA positive samples were typed by Restriction Fragment Length Polymorphism (RFLP) according to Bernard et al., 1994. Briefly, aliquots of the PCR products obtained using the degenerate consensus primers MY09–MY11, targeting a region of approximately 450 bp in length in the L1 ORF of the viral genome, were mixed with 7 different restriction enzymes (Bam HI, Hae III, Dde I, Pst I, Hinf I, Sau III, and Rsa I) in separate reactions. The digestion products were separated by electrophoresis in a 3% agarose gel and the pattern obtained was compared with published data.Statistical analysis: we used the software InfoStat version 2011, with a significance level of 5% (95% CI) (InfoStat, computer program 2011)[22].Chi-square (χ2) tests were conducted to determine the possible associations between presence, type and location of the lesions, cytological classification, history of tobacco smoking habit, alcohol consumption, age, gender, HPV and other risk factors.
3. Results
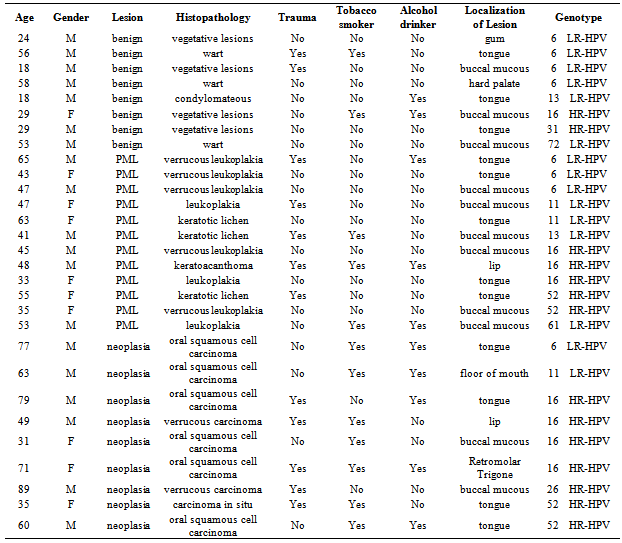
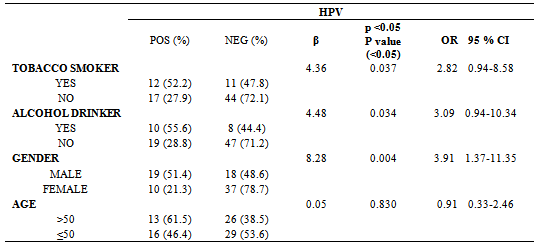
- The patients were classified into four groups, as follows:Control group: This group comprised 30 individuals, 8 males and 22 females, mean age: 41.8±17.1 years (range, 18-81). Most controls were non tobacco smokers and non drinkers ((23/30 and 25/30, respectively). Inclusion criteria were: immunocompetent status and absence of any detectable lesions in the oral mucosa.Patients with benign lesions: The mean age of the 9 patients of this group (1 female, 8 males) was 34.0±16.8 years (range, 18-58 years); 2 patients were tobacco smokers, 2 reported regular alcohol consumption and 1 both habits. The most frequent locations were the buccal mucosa (n=3) and tongue (n=3), followed by uvula (n=1), hard palate (n=1) and gum. The distribution by cytology showed: grade II (n=7) and grade III (n=2).Patients with potentially malignant lesions (PML): The mean age of the 29 patients (16 females, 13 males) was 52.6±14.7 years (range, 29-76 years). Four patients were tobacco smokers, 4 reported regular alcohol consumption and 3 both habits. The most frequent location was tongue (n=18), followed by oral mucosa (n=8), hard palate (n=1), mouth floor (n=1) and lip (n=1). The results of cytology showed: grade II (n=10) and grade III (n=19).Patients with neoplasia: The mean age of the 16 patients (8 females, 8 males) was 59.6±19.7 years (range, 23-89 years), 10 were smokers; 7 reported alcohol consumption and 5 both habits. The most frequent location was tongue (n=8), followed by the oral mucosa (n=3), retromolar trigone (n=1), lip (n=1), gum (n=1), hard palate (n=1) and mouth floor (n=1). The distribution based on the cytological findings was: grade III (n=7), grade IV (n=3) and grade V (n=6).HPV was detected in 88.89% (8/9) of the samples from oral benign lesions, 41.38% (12/29) of oral PML samples and 56.25% (9/16) of oral neoplasias. In samples from the control group, HPV was not detected.Table 1 shows the characteristics of HPV-positive patients and the genotypes detected. The most prevalent were genotypes 16 and 6. Together, these two genotypes reached 55% of the total number of patients and both were identified within the 3 groups of patients. Figure 1 shows the percentage of detection of HR-HPV and LR-HPV in the different groups.Table 2 shows the correlation between HPV and the different variables. A significant association of HPV with tobacco smoking habit, alcohol drinking and male gender was detected, but no association was found in patients over 50 years of age. With these data, we performed a statistical analysis between the different variables including the finding of HPV, versus clinical and histopathological diagnosis.
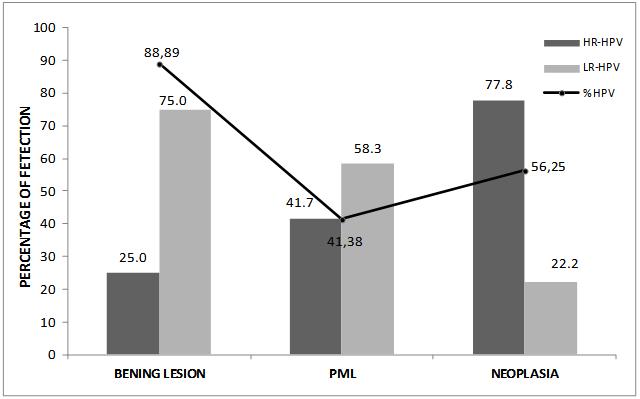 | Figure 1. Detection of HR-HPV and LR-HPV in the different lesions |
|
|
|
4. Discussion
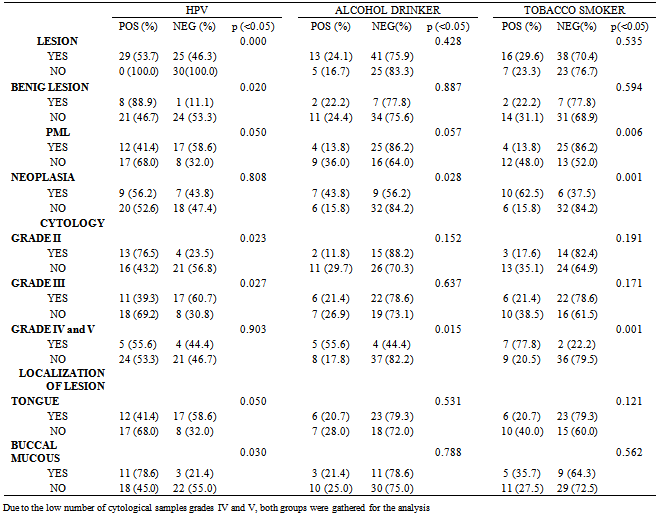
- Understanding the role of HPV in oral carcinogenesis is hard due to the different frequencies of HPV infection in potentially malignant lesions and in oral cancer[23,24].The percentage of HPV detection in our study was similar to data reported in other publications[25,26], likewise, no significant differences were observed between the detection of the different genotypes, HR-HPV and LR-HPV (14 vs 15 respectively). However, we can observer that the high-risk types were found mainly in malign lesions, while the low risk types were detected mainly in benign lesions. This becomes evident when analyzing the data shown in Figure 1, where is observed that HR-HPV was detected in 77.8% of cases of neoplasia (HPV+), while that LR-HPV was detected in only 22.2% of patients in this same group. An reverse situation is observed in the group of patients with benign lesions, showing clearly that HR-HPV is associated with increased severity of the injury. Like in previous publications, HPV-16 was the HR-HPV genotype most frequently found[27,28] and 52 HR-HPV the second, but unlike other studies, genotype 18 was not detected at all in our work[28].The presence of oral trauma and/or candida was included within the clinical histopathological variables analyzed; we did not find a significant association with HPV, history of alcohol intake, tobacco smoking, gender or age. Also, like other authors, our study did not show significant associations between HPV and patient´s age[5,29].Unlike other authors who have reported higher rates of HPV detection in patients without history of tobacco or alcohol intake[30,31], our study showed that patients who smoked tobacco or drinked alcohol were 3 times more likely to have infections by HPV (Table 2).Significant associations of HPV with cytological grade II, and tobacco and alcohol with cytological grades IV and V was in agreement with the finding of these risk factors for benign and malignant lesions, respectively. (Table 3).On other hand, we observed an negative association between HPV with cytological grade III and tobacco smokers with PML, both with OR less than 1 (0.29 and 0.17) indicated that cytology grade III and PML are less frequent in those patients with HPV infection and tobacco smoking history respectively[32].As in other publications, the more frequent location of the lesions was the tongue[25,33] accomplishing 53.70% of the cases, followed by 25.93% in the buccal mucosae. In the statistical analysis, both tongue and buccal mucous showed no significant association with other groups (benign lesions, PML and neoplasias). The lesions of the buccal mucous presented significant association with HPV, which is in agreement with data previously reported by other authors on the basis of the epitheliotropic nature of the virus (table3) [11,12].Our results showed that tobacco smoking and alcohol drinking have more influence than HPV on the development of neoplasic lesions. However the 56.2% of the neoplasias were positive for HPV (Table 3), and the percentage of HR-HPV detection increases with the severity of the lesions, suggesting the possible involvement of HPV in the malignant processes (Figure 1). Perhaps, may be that HR-HPV alone is not able to significantly increase the risk of developing malignant lesions of the oral cavity, but when in conjunction with other factors such as tobacco and alcohol, it develops an important role in developing these lesions.Many studies have confirmed that HPV is the causative agent of cervical cancer, but its role as etiologic agent of oral cancer needs to be further studied to achieve accurate conclusions.
Funding
- This study was supported by grants from the Fundación Roemmers subsidio 2012-2014 and Ministerio de Ciencia y Técnica de la Provincia de Córdoba-Argentina. PIO-MincytCba. 2011-2013. Ref. Res. [MINCYT Cba. Nº 170/2011].
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML