-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Microbiology Research
p-ISSN: 2166-5885 e-ISSN: 2166-5931
2013; 3(5): 185-196
doi:10.5923/j.microbiology.20130305.04
Control of Gardenia Leaf Spot and Bud Rot Diseases Using Some Natural Plant Oils
M. A. Mostafa1, M. M. Alawlaqi2, Nour El-houda A. Reyad3
1Pl. Pathol. Dept., Fac. Agric. Cairo Univ. Current address: Bio. Dept. Faculty of Science, Jazan Univ, Saudi Arabia
2Bio. Dept. Faculty of Science, Jazan Univ, Saudi Arabia
3Pl. Pathol. Dept., Faculty of Agric. Cairo Univ, Egypt
Correspondence to: M. M. Alawlaqi, Bio. Dept. Faculty of Science, Jazan Univ, Saudi Arabia.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
This study was conducted to through light on the most important fungi affected gardenia (Gardenia jasmenoides Ellis) plant with leaf spot and bud rot diseases and the effect of some plant essential oils as safe management against these fungi in vitro and in vivo. Isolation trials from infected gardenia plant tacken from Giza governorate during 2010-2011 growing season revealed eleven fungal species related to eleven genera. Botrytis cinerea, Alternaria alternata, Pestalotia langloissii and Cladosporium sp. were the most dominant fungi. These four isolates were differed in there pathogenic capabilities depending on the infected plant part, B. cinerea was exhibited the highest percentage of rotted buds while A. alternata and P. langloissii were only infected the leaves. A. alternata was exhibited the highest disease severity. Among twenty plant essential oils tested in vitro, Cumin (Cuminum cyminum) oil was the most effective one, completely inhibited the mycelial growth of the tested fungi at 500 ppm. Generally spraying gardenia plant by cumin oil at 2500 ppm. mixed with clove oil at 5000 ppm. concentration was the best treatment that significantly decreased the disease incidence under greenhouse conditions.
Keywords: Gardenia, Fungi, Leaf Spot, Bud Rot
Cite this paper: M. A. Mostafa, M. M. Alawlaqi, Nour El-houda A. Reyad, Control of Gardenia Leaf Spot and Bud Rot Diseases Using Some Natural Plant Oils, Journal of Microbiology Research, Vol. 3 No. 5, 2013, pp. 185-196. doi: 10.5923/j.microbiology.20130305.04.
Article Outline
1. Introduction
- Production of ornamental plants is a considerable sector of the economical agricultural income. Nowadays, the indoor plants became necessary to overcome serious problems of air and environmental pollution, particularly in the closed place or small apartments. The Gardenia genus is considered one of the most important cutting flower plants which includes over 200 species, with the most important ones Gardenia jasminoides Ellis (native to china) and Gardenia thubergia L.F (White gardenia, native to South Affrica). This genus is belonging to Rubiaceae (Coffee) family.The richly scented Gardenia jasminoides Ellis is suffering from several diseases such as leafspot[1],[2],[3],[4],[5],[6],[7],[8],[9] and[10], Flower blights and bud rots[4],[7],[11] and[12], and stem canker[1],[13],[14],[15],[16], and[17].Gardenia leaf spot caused by Alternaria alternata and Pestalotia langloissii as well as bud rots caused by Botrytis cinerea become to be serious on G. jasminoides under Egyptian greenhouses, Particularly in moist conditions where the disease can lead to heavy defoliation despite the excessive and indiscriminate use of synthetic fungicides.The excessive and indiscriminate use of synthetic fungicides are cause many hazards to humans and animals due to their possible carcinogenicity, teratogenicity, high and acute toxicity, long degradation periods and environmental pollution[18], Also, spraying these materials on the foliages of gardenia plant results in a decrease in plant quality because of its deposits on the leaves. So, the exploitation of natural substances such as plant essential oils is urgently needed as alternatives to these synthetic fungicides[19], as they are easily decomposable, not environmental pollutants and posses no residual or phytotoxic properties[20],[21],[22], and[23].Essential oils are volatile, natural and complex compounds characterized by a strong odour and are formed by aromatic plants as secondary metabolites. Production of these oils by plants is believed to be predominant a defense mechanism against pathogens[24], and indeed, they have been shown to possess antifungal properties both in vitro and in vivo[23],[25] and[26]. The complexity plant essential oils relates to their highly contents from natural components that ranging between 20 and 60 components at quite different concentrations. Among these large numbers of components there are two to three components called the major components are find at fairly high concentrations 20-70 % compared to others components present in trace amounts[27], Although the major components are reflect quite well the biophysical and biochemical features of the essential oils. it is difficult to correlate the fungitoxic activity to single compound or class of compounds[28], where the synergistic or antagonistic effect of one compound in minor percentage in the mixture must be considered, as each of the essential oil components has its own contribution on biological activity of the oil[13]. Generally, inhibition of fungal growth by essential oils often involves induction of changes in cell wall composition[29], plasma membrane disruption,mitochondrial structure disorganization[30], and interference with enzymatic reactions of the mitochondrial membrane, such as respiratory electron transport, proton transport and coupled phosphoration steps[31]. Plants have evolved physiological and biochemical mechanisms, including increases in the activities of oxidative and reductive enzymes associated with a biotic and biotic factors[32]. This response has been observed by several investigators related to plant defense[33] and constitutes an evolutionary strategy of plants for defending themselves against pathogens. The objective of this study is to through light on the most important fungi affecting gardenia (Gardenia jasminoides Ellis) plants causing leaf spot and bud rot diseases and the effect of some plant essential oils as safe management against these fungi in vitro and in vivo.
2. Materials and Methods
2.1. Isolation, Purification and Identification of the Associated Fungi
- Gardenia plants (Gardenia jasminoides Ellis) grown under greenhouse conditions in commercial nurseries located at Giza governorate were suffered from leaf spot and bud rots diseases all over the season. During February, April, July and November of 2010-2011 growing season, diseased plant were selected and transferred in their pots to the Lab of Plant Pathology Dept. Fac. Agric. Cairo University.Isolation trials were carried out during the previously mentioned periods on potato dextrose agar medium (PDA). Spots with different sizes and colours were carefully cut using sterilized forceps. The fragments were surface sterilized using 1% sodium hypochlorite. Under aseptically conditions, fragments were transferred to place onto the surfaces of sterilized PDA medium in Petri-dishes (9 cm. in diam.). Petri-dishes were then incubated at 23° ±1°C for 3 days. The emerged fungi were picked up and subcultured onto fresh PDA medium. Fungi were purified using hyphal tip or single spore technique adopted[34]. Purified fungi were identified according to their morphological characters using the keys given by[35],[36] and[37].Occurrence and frequency of fungi isolated at the end of the incubation period were determined according to the following formula:% X = N/T x 100Where:% X = frequency of the fungus.N = Number of colonies for the fungus.T = Total number of fungal colonies for the isolated fungi.
2.2. Pathogenicity Testes
- Pathogenicity testes were conducted for the most dominant fungi isolates namely, Botrytis cinerea, Alternaria alternate, Pestalotia langloissii and Cladosporium sp. Each isolate was separately grown on PDA medium at 23° ±1°C for 7 days. A spore suspension was prepared by adding 10 ml. of distilled water to each plate and tapering the spores using a camel hair brush. The spore concentration was adjusted to 5X10³ conidia/ml. using a haemicitomiter. Under greenhouse conditions, where the degree of temperature was 25º±2ºC and relative humidity (RH) was 65%, healthy gardenia plants (6-month old) grown in pots 20 cm in diameter containing autoclaved peatmos were used in this investigation. Before artificial inoculation, a drop of Tween 20 was added to the spore suspension as a wetting agent. Spore suspension of any of the four tested fungi was sprayed on both leaf surfaces and buds of the plant with an atomizer and each plant received 20 ml of spore suspension. Treated plants were covered with polyethylene bags to maintain high relative humidity for 24h then removed. Control plants, were similarly treated only by sterile distilled water mixed with a drop of Tween 20. Three plants were used for each particular treatment. Plants were observed daily for three weeks following inoculation looking for leaf spot and bud rot symptoms.Rotted buds on each treated plant were determined after 7 days following inoculation period as percentage of rotted buds to the healthy one. Whereas, for leaf spot determination, disease index was measured within three weeks following inoculation. Areas of visible symptoms were scored for disease index on a scale of 4 points as follows:0 = no symptoms.1 = few scattered lesions covering about 1-10% of the leaf.2 = spots covering about 11-25% of the leaf.3 = spots coalescing and covering about 26-50% of the leaf.Disease index was converted according to the equation suggested by[38] as follows:Disease index % = Σ n/N x 100Where:(n) Is the number of leaves in each numerical grade(r) and (N) is the total number of inoculated leaves multiplied by the maximum numerical grade (4).
2.3. Effect of some Plant Oils on the Linear Growth of the Three Tested Fungi a- Source of Plant Oils
- Several essential oils were tested for their antagonistic effects against the three tested fungi i.e., B. cinerea, A. alternata, and P. langloissii the causal agents of leaf spot and bud rots of gardenia plant either in vitro and in vivo. The tested oils were from. clove ( Syzigium aromaticum L.), anise (Pimpinella anisum ), peppermint ( Mentha piperita ), cumin (Cuminum cyminum ), coriander ( Coriandrum sativum ), French basil ( Ocimum basilicum ), local basil (Ocimum kilimandscharium), caraway (Carum carvi), thyme (Thymus vulgaris), fennel (Foeniculum vulgare), Egyptian geranium (Pelargonium graveolens), sage (Salvia officinalis), rosemary (Rosmarinus officinalis), chamomile (Ormenis mixta), parsley (Petroselinum crispum), marjoram (Origanum vulgare), dill (Anethum graveolens), celery (Apium graveolens), eucalyptus (Eucalyptus citriodora) and tagets (Tagetes patula). These plant oils were obtained from Medicinal and Aromatic Pl. Res. St. El-Quanter El- Khairiah, Qulubyiah governorate, Hort. Res. Inst., Agric. Res. Center (ARC), Giza, Egypt.
2.4. In Vitro Effect of the Individual Plant Oils on the Linear Growth of the Tested Fungi
- The minimum inhibitory concentration of the tested plant oils against the three tested fungi was conducted using a poisoned plate technique or the agar dilution method described by[39]. The oils were added in a separated mean to sterile melted PDA medium containing drop of Tween 20 to produce the concentrations 500, 750, 1000, 1500 and 3000 ppm. The resulting PDA solutions were immediately poured into sterilized Petri-dishes (9 cm. in diam.) at the rate of 20 ml/plate. Dishes were inoculated at the center with 4 mm mycelial disc cut from the periphery of 7- day-old culture of any of the three tested fungi. The inoculated plates were incubated at the optimum temperature for each fungus, i.e. 20°C for B. cineria and 25°C for A. alternata and P. langloissii. Three replicate dishes were used for each treatment. Plant oil free PDA medium with drop of Tween 20 was used as control. The diameter of the developed colonies was measured when the mycelial growth of the fungus covered the plates of check treatment. The inhibition in mycelial growth rate was calculated according to formula suggested by[40]. as follows:I=C-T/C x100Where:(I) is the inhibition percentage of mycelial growth.(C) is the mean colony diameter (mm) of the control set.(T) is the mean colony diameter of treatment sets.
2.5. Effect of Mixing Cumin, Clove, Thyme, Peppermint and Anise Oils on the Growth of the Tested Fungi
- Five of the most effective plant oils were selected to study their toxicity on the tested fungi when mixed together, i.e. cumin (Cuminum cyminum), thyme (Thymus vulgaris), anise( Pimpinella anisum ), peppermint ( Mentha piperita ), and clove ( Syzigium aromaticum L.) oils, where the mixture was conducted between each two oils at the rate of 1:1 (500:500 ppm.), 1:2 (250:500 ppm.) and 2:1 (500:250 ppm) concentrations. The toxicity of each mixture was tested by the method mentioned above and described by[38]. Three replicates were used for each treatment. Plant oil free PDA medium with a drop of Tween 20 was used as control. Also, mycelial parts of fungi which could not grow during the assay were transferred into sterile essential oil-free PDA media and then observed for a week to determine the fungicidal or fungistatic effect for each mixture of plant oils. The days needed for mycelium reactivation for each fungus were also calculated.
2.6. Effect of Cumin Oil Individually or in Mixture with Anise or Clove Oil on Percentage of Mycelial Growth and Germinated Sclerotia of Botrytis Cinerea
- Sclerotia of Botrytis cinerea the causal organism of gardenia bud rot disease were dipped in cumin oil at 500 ppm. as individual treatment and on the mixure of cumin and anise or clove oils at the rate of 1:2 (i.e. 250:500 ppm.) concentrations for 15 minutes. Sclerotia were removed with the help of sterilized fine forceps, placed on sterilized blotting paper to remove access of water and each sclerotium placed at the center of PDA medium. For control, sclerotia were only dipped in sterilized water then placed on PDA medium. Petri-dishes were incubated at the optimum temperature (20°C) for the fungus. Three replicates were used for each treatment. The averages of the linear growth in mm were calculated when the mycelium reached its maximum growth in the check treatment.On the other hand, the sclerotia were centrifuged with the three oils at the same concentrations mentioned above as described by[41] at 1000 rpm for 15 min. and decanted to remove mycelial fragments. Sclerotia were removed with the help of sterilized fine forceps, placed on sterilized blotting paper to remove access of water and then placed on PDA medium. For control, sclerotia were centrifuged at 1000 rpm for 15 min. with sterilized distilled water only. Five sclerotia were kept for each treatment and replicated three times. Percentages of germinated sclerotia were calculated 24h following incubation at the optimum temperature i.e. 20°C.
2.7. Effect of Cumin Oil Individually or in Mixture with Anise or Clove Oils on Controlling Bud Rot Caused by B. cinerea and Incidence of Leaf Spot Disease Caused by A. Alternata and P. Langloissii
- Healthy Gardenia plants (6-month old) grown in sterilized pots (25cm in diam.) containing peatmos were used to study the effect of cumin oil individually and mixurally with anise or clove oil on controlling the infection caused by the three tested fungi under greenhouse conditions. The tested oils were diluted to 5000 ppm for cumin oil treatment and 2500:5000 ppm for the mixtures, where the concentration of cumin oil in the mixture was 2500 ppm, meanwhile both clove and anise oils were mixed by 5000 ppm for each. The tested oils sprayed onto the upper leaf surfaces to run-off using an atomizer 24h before inoculating the plants with a spore suspension of each fungus (5.0x10ł conidia/ml). Control treatment consisted of sterilized distilled water containing 0.5 % Tween 20. The percentage of rotted buds was assessed 8 days following the inoculation. Meanwhile, the disease index of leaf spot symptoms was assessed 3 weeks following inoculation. Three pots were used for each treatment.
2.8. Effect of Cumin Oil Individually and in a Mixture with Anise or Clove Oil on Controlling Bud Rot and Leaf Spot Diseases Under Natural Infection Under Greenhouse Conditions
- To study the effect of cumin oil individually or in mixture with anise or clove oil under greenhouse conditions, healthy Gardenia plants (6-month old) grown in sterilized pots (25cm in diam.) containing peatmos were divided into three groups. At the beginning of March, 2007 the first group sprayed by cumin oil at 5000 ppm concentration, the second group sprayed by a mixture of cumin oil at 2500 ppm and anise oil at 5000 ppm concentration and the third group sprayed by a mixture of cumin oil at 2500 ppm and clove oil at 5000 ppm concentration. The plants were sprayed randomly by atomizer each week through 2007 season. Three replicate pots were used for each treatment. Control treatment consisted of healthy plants sprayed with sterilized distilled water containing 0.5 % Tween 20. The rotted buds were observed at May 2007 and the percentage of rotted buds was calculated as mentioned under pathogenicity test. The resulting leaf spots symptoms were observed at September 2007 and the disease index was calculated as mentioned before.
3. Results and Discussion
3.1. Isolation, Purification and Identification of the Associated Fungi
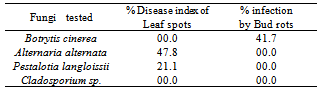
- In the present study (Table 1) Isolation trails, from naturally infected gardenia plant by leaf spot and bud rot symptoms collected from greenhouses located at Giza governorate resulted in the presence of eleven fungal species belonged to eleven fungal genera. These fungi were identified as Botrytiscinerea, Alternaria alternata, Pestalotia langloissii, Cladosporium sp., Stemphylium sp., Phomopsis sp., Myrothecium roridum, Conitherium sp., Fusarium oxysporum., Botryodiplodia sp. and Trichoderma viride. These isolates were differed in their occurrence and frequencies according to the infected plant part and the seasonal isolation periods. Generally, B. cinerea, A. alternata, P.langloissii and C. sp. were the most prevailing fungi. B. cinerea was isolated only from the rotted buds formed in spring season at the rate of 77%, where it was recorded the highest frequency of occurrence during this period. A. alternata, P.langloissii and C. sp. were isolated from both infected leaves and buds through all the isolation periods except for A. alternata which not isolated in spring season. The heights frequency of C. sp. was recorded in winter season at the rate of 50% and 75% from infected leaves and buds, respectively followed by spring season by 57.1% and 15.4% from leaves and buds, respectively. Meanwhile, the highest frequency of A. alternata and P. langloissii were recorded in summer season. The corresponding percentages were 40% and 15.4% for A. alternata and 30% and 30.8% for P. langloissii from infected leaves and buds, respectively.According to the available literature, the recorded causal organisms of leaf spot and/or bud rot diseases of Gardenia jasminoides Ellis in Egypt were Fusarium solani, Nigrospora sp., Rhizoctonia solani on the flowers and Myrothecium roridum on the leaves[10], but in other countries Numerous investigators have shown that leaf spot and bud rots diseases of Gardenia jasmenoidesEllis are caused by different fungi such as Botrytis primer[12], Botrytis cinerea[11] and[7], Alternaria alternata[3], Pestalotia langloissii[2], Pestalotia spp.[42], Myrothecium roridum[1],[6] and[7], Rhizoctonia spp.[7], Cercosporidium okinawaense[42], Phyllosticta gardiniicola[9], and[4], Phyllosticta sp.[7], Mycosphaerella luzonensis[8], and Colletotrichum gloeosporides[5], and[9]. This higher numbers of the recorded fungi that associating with gardenia plant may be attributed to the physiobiochemical processes in the plant.
|
3.2. Pathogenicity Testes
- Pathogenicity tests by The most prevailing fungi isolated from gardenia plants namely, B. cinerea, A. alternate, P. langloissii and Cladosporium sp., indicated that these isolates were differed in there pathogenic capabilities to leaves and buds of the plant (Table 2.) The pathogenic fungi were Botrytis cinerea, Alternaria alternata and Pestalotia langloissii but Cladosporium sp was non-pathogenic. B. cinerea, A. alternata and P.langloissii were specialized in their pathogenic capabilities depending on the infected plant part. B. cinerea was only pathogenic to the plant buds, where it exhibited the highest percentage of rotted buds, being 41, 7% .Meanwhile, A. alternata and P. langloissii were pathogenic to the leaves. A. alternata exhibited the highest disease index, being 47.8% while, P. langloissii was the lowest one in this respect, being 21.1%.
|
3.3. In Vitro Effect of the Individual Plant Oils on the Linear Growth of the Tested Fungi
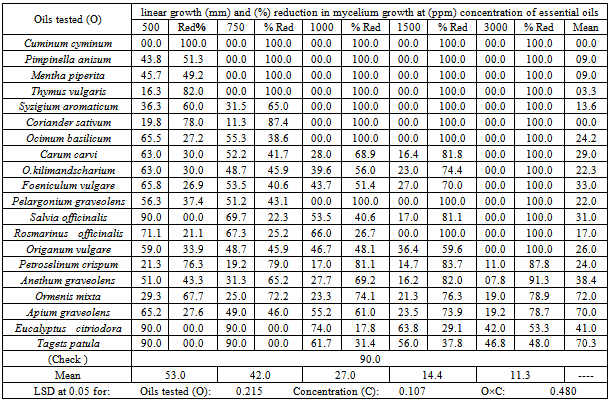
- Data obtained (Tables 3,4 and 5) clear that as the concentration of each oil increase, linear growth of the tested fungi decreased, and this effect differed according to the oil type. Cumin oil was the most effective one, completely inhibited the mycelial growth of the tested fungi at 500 ppm concentration or more, while clove, anise, thyme, coriander, French basil and peppermint oils at concentrations ranged from 750 to 1500 ppm according to the fungus type occupied the second position in this respect. Where, clove, anise, thyme and peppermint oils at 750, 1000, 1500 and 1500 ppm concentrations, respectively completely inhibited the mycelial growth of A. alternata. anise, peppermint and thyme oils at 750 ppm concentration as well as clove, French basil and geranium oils both at 1000 ppm also, rosemary oil at 1500 ppm concentration completely inhibited the mycelial growth of P. langloissii. Meanwhile, anise, thyme, peppermint, clove, coriander and French basil oils at 1000 ppm concentration completely inhibited the mycelial growth of B. cinerea.On the other hand, caraway, local basil, fennel, marjoram, rosemary and geranium oils at 3000 ppm completely inhibited the mycelial growth of the tested fungi. Meanwhile, celery, parsley, chamomile, eucalyptus, tagets oils were the lowest activity. Where each of them only resulted in reduced of mycelial growth of the tested fungi till the highest tested concentration 3000 ppm. These results are in harmony with several workers.[44] arranged the active parts of volatile oils according to the antimicrobial activities in the decreasing orders as follows: Aldehyde, Phenols, Alcohols, Ketons and Hydrocarbons.[45] mentioned that the pure essential oilscompletely inhibited the mycelial growth of many pathogenic fungi, and the fungal sensitivity to the previous essential oils differed in their effect from one fungus to another, this might be due to the capability of essential oils to penetrate into the fungal cells. According to[46], the activity of the plant essential oils was divided depending on the minimum inhibitory concentration (MIC) to three divisions i.e. strong (MIC more than 500 ppm), Moderate ( MIC from 600 ppm to 1600 ppm ) and weak (MIC more than 1600 ppm). So, the results obtained from the present study of cumin oil on the fungi tested lead to group the oil into a strong effect category, where it is completely inhibited the mycelial growth of the tested fungi at 500 ppm concentration The inhibitory effect of cumin oil might be attributed to the presence of the cuminaldehyde. Anise, peppermint, clove, coriander, thyme and French basil oils were showed a moderate affect. Where, they completely inhibited the mycelial growth of the fungi tested at concentrations ranging from 750 to 1500 ppm. ,except for coriander oil which showed a week effect only on A. alternata where it is completely inhibited the mycelial growth of the fungus at 3000 ppm concentration., This might be due to the presence of the great amount of phenolic and alcohols substances like thymol in thyme oil, eugenol in clove oil, menthol and carvacrol besides menthyl acetate ester in peppermint oil, chavicol beside linalool alchohol, methyl chavicol and camphen compound in French basil oil[47],[48] and[49]. The other tested oils i.e. caraway, local basil, fennel, marjoram, rosemary, geranium, celery, parsley, chamomile, eucalyptus, tagets oils were the lowest activity. Where each of them only resulted in a reduction of the mycelial growth of the tested fungi till the highest tested concentration 3000 ppm., except for rosemary and geranium oils which showed a moderate affect only on P. langloissii where they completely inhibited the mycelial growth of the fungus at 1500 and 1000 ppm concentration respectively. This week activity of the previously mentioned oils might be due to their poorness in phenolic compounds for example local basil contains the same compounds in French basil but in small quantities also, marjoram oil is very poor in its phenolic compounds except cineol compound which act as antifungal agent in a great amounts reaching to 30.1% but it was poor in other phenolic compounds[26],[47],[48] and[50]. The MIC and toxicity concentrations of the essential oils varied from study to study and this is probably due to the different methods of extraction of the essential oils and different sensitivity of the tested fungi[51].
3.4. Effect of Mixing Cumin, Clove, Thyme, Peppermint and Anise Oils on the Growth of the Tested Fungi
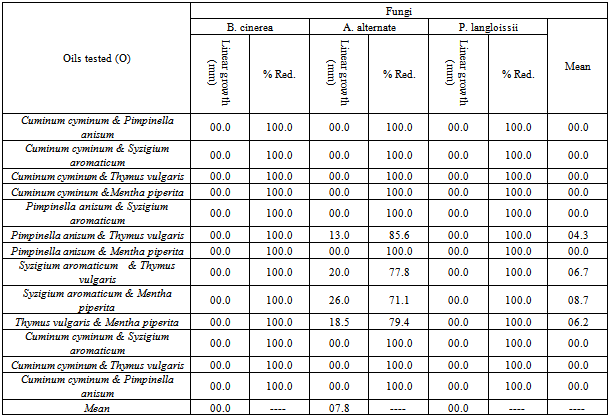
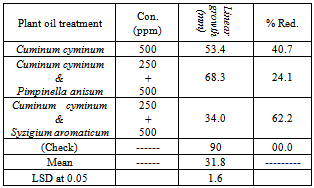
- The mixture between the tested oils completely inhibited the mycelium growth of the tested fungi except for the mixture of (anise & thyme), (clove & thyme), (clove & peppermint) and (thyme & peppermint) where they only resulted in the reduction in mycelial growth of A. alternata from 90 mm in the absence of oil (check) to 85.6; 77.8; 71.1 and 79.4 mm, respectively. (Table 6).[52] mentioned that combinations of hydrosol, oleoresin, ground material and essential oils may provide an efficacious mixture for the inactivation of pathogenic and spoilage microorganisms in plant and foods.
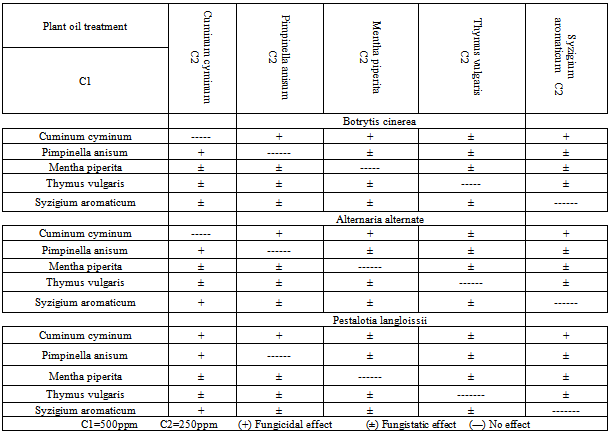
3.5. Fungicidal and fungistatic Effects of the Tested Oils Individually and in Mixture on the Fungi Tested
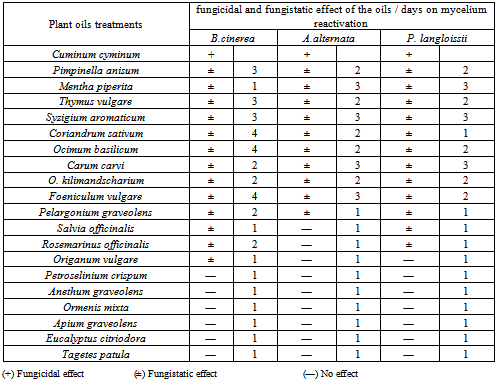
- Cumin oil as individual treatment had a fungicidal effect on the tested fungi. Meanwhile, the other individual oils tested had a fungistatic effect and the days needed for mycelium reactivation ranged from 1 to 4 days according to the fungus type. (Table 7). On the other hand the mixture of cumin oil at 500 ppm with any of the tested oils at 500 ppm gave a fungicidal effect on the tested fungi except for A. alternata where in case of the treatment with the mixture of (cumin & thyme) and (cumin &peppermint) a fungistatic effect was obtained and the mycelium reactivated through four days. this might be related to the differences in the ultrasruction of the fungus conidia .[45] mentioned that the pure essential oils completely inhibited the mycelial growth of many pathogenic fungi and the fungal sensitivity to the previous essential oils from one fungus to another, this also might be due to the capability of essential oils to penetrate into the fungal cell.On the other hand, reduced the concentration of cumin oil to 250 ppm and fixed the concentration of any of the tested oils in the mixture at 500 ppm had a fungicidal effect only in case of the mixture of (cumin & anise) and (cumin & clove) oils on both B. cinerea and P. langloissii. Meanwhile, cumin oil at 250 ppm mixed with clove oil at 500 ppm had a fungicidal effect on and A. alternata. The other mixtures had a fungistatic effect on the tested fungi. While, fixed the concentration of cumin oil at 500 ppm and reduced the concentration of any of the tested oils in the mixture to 250 ppm had a fungicidal effect on the tested fungi (Table 8).[53] mentioned that the fungicidal activity of some essential oils constitutes such as trans-2-hexanal and citral aldehydes may be ascribed to the high electrophilic properties of the carponyl group adjacent to the double bond that make these compounds particularly reactive with neucleophiles, such as protein sulfhydryl and amino groups of the pathogen.[54] mentioned that essential oils inhibited the growth of fungi either temporarily (fungistatic) or permanently (fungicidal). [55] reported that most essential oils are fungistatic effect than fungicidal. In this study, the fungicidal effect was only recorded for the cumin oil which contains cuminaldehyde as the major component.
|
|
|
|
|
3.6. Effect of Cumin Oil Individually or in Mixture with Anise or Clove Oil on Percentage of Mycelial Growth and Germinated Sclerotia of B. Cinerea
|
|
3.7. Effect of Cumin Oil Individually or in Mixture with Anise or Clove Oils on Controlling Bud Rot Caused by B. Cinerea and Incidence of Leaf Spot Disease Caused by A. Alternata and P. Langloissii
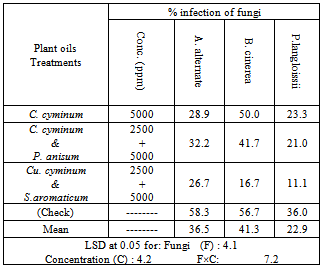
- The experiment of spraying gardenia plant by cumin oil as individual treatment or in a mixture with clove or anise oil on the percentage of reduction in the rotted buds caused by B.cinerea and the disease index of leaf spot disease caused by A.alternata and P.langloissii under artificial inoculation conditions showed that cumin oil at 2500 ppm mixed with clove oil at 5000 ppm concentration exhibited the heighst reduction percentage in the disease incidence by the three tested fungi under artificial inoculation. Where the treatment resulted in decreasing the incidence of gardenia bud rot disease caused by B.cinerea from 58.3% in (check) treatment to 16.7% and also, decreasing the incidence of gardenia leaf spot disease caused by A.alternata and P.langloissii to 26.7% and 11.1%, respectively compared with the (check) treatment 56.7 and 36.0%, respectively. Cumin oil at 2500 ppm mixed with anise oil at 5000 ppm concentration treatment followed the previous mentioned treatment. Meanwhile, cumin oil at 5000 ppm concentration was the lowest one in this respect (Table 11).
|
3.8. Effect of Cumin Oil Individually and in a Mixture with Anise or Clove oil on Controlling bud Rot and Leaf Spot Diseases under Natural Infection in Greenhouse
- The in vivo treatments by the same oils and by the same concentrations were showed lower efficacy than those conducted under artificial inoculation conditions in the greenhouse but in general, cumin oil at 2500 ppm mixed with clove oil at 5000 ppm concentration exhibited the heighst percent of reduction in the disease incidence of gardenia bud rot and leaf spot disease Where, the treatment resulted in decreasing the incidence of gardenia bud rot disease from 43.3% in (check) treatment to 23.4% by 46% efficacy and also, decreasing the incidence of gardenia leaf spot disease from 41.7% in (check) treatment to 25.0% by 40.0% efficacy .followed by cumin oil at 5000 ppm. where, it reduced the incidence of gardenia leaf spot disease from 41.7% in (check) treatment to 33.3% by 20.1% efficacy (Table 12).[55] reported that very few studies have analyzed enough essential oils and biological endpoints to determine whether there is a specificity for different effects according to deferent oils or not . Clearly, it has been shown by[56] and[57], that the essential oils presented a specificity in the amplitude, but not in the mode of action, of the biological effects, i.e. cytotoxicity, cytoplasmic mutant induction, gene induction and antigenotoxicity effects. However, they did exhibit a specificity of the mode of action concerning production of ROS, probably due to differences in their actual composition corresponding to differences in compartmentation of the oxidative stress.[58] concerning antigenotoxicity, the essential oils showed the same protective activity. However the mode of action of protection differed, not according to the type of oil, but according to the mutagens, i.e. to the type of lesions induced and thus, to the type of their enzymatic recognition and processing lead to translational synthesis or late apoptosis/necrosis[56] and[57].
|
4. Conculutions
- The authors believe that the fungi isolated from Gardenia plants suffering from leaf spot and bud rot symptoms collected from greenhouses located at Giza governorate, Egypt were differed in their occurrence and frequencies according to the infected plant part and the seasonal isolation periods. Pure essential oilscompletely inhibited the mycelial growth of many pathogenic fungi, and the fungal sensitivity to the essential oils tested differed in their effect from one fungus to another, this might be due to the capability of essential oils to penetrate the fungal cells . Combinations of essential oils may provide an efficacious mixture for the inactivation of pathogenic and spoilage microorganisms in plant and foods. Fungicidal and fungistatic effects of the oils tested individually and in mixture on the fungi tested were illustrated. Most essential oils are of fungistatic effect than fungicidal. So, it is recommended to use natural oils for controlling the diseases under study.
References
| [1] | Barrett, J.T and Hardman, D.A (1947). Myrothecium leaf spot and canker of gardenia. Phytopathol. 12 (5) 360-361. |
| [2] | Shoemaker, R.A and Straby, A.E. (1965). Pestalotia langloissii on gardenias. Pl. Dis. Reptr 49 (10), 832-833. |
| [3] | Gupta, G.H and Prasad, B. (1983). A new host of Alternaria alternata. Indian J. Mycol. Pl. Pathol. 13 (3), 359-360 |
| [4] | Kamal, R; Singh, R.P and Shukla, D.N. (1983). Fungi of Gorakhpur. Indian J. Mycol. Pl. Pathol. 12(2), 160-164. |
| [5] | Ciccaron, C. (1958). An anthracnose due to Colletotricum on Gardenia leaves. Informator- phytopathologico. 35 (11), 55-58. Cited in http:// www. Eul. |
| [6] | Cappelli, C. (1996). Attacks by (G. jasminoides) in Italy. Informator-Phytopathologico. 46 (10), 47-49. Cited in http:// www. Eul. Edu. Eg. |
| [7] | Dreistadt, S.H. (2001). Integrated pest management for floriculture and nurseries. University of California, Division of Agriculture and Natural Resources. Publication, pp. 3402. |
| [8] | Kobayashi T; Nakashima C. and Nishijima T. (2003). Notes on some plant inhibiting fungi collected from the nansei islands. Mycosci. 44, 473-479. |
| [9] | Zheng-shiweii and Lao-chong. (2004). Occurrence and control of disease of Gardenia jasminoides in Cixi. J. Zhejiang forestry science and technology. 24 (2), 53-54. |
| [10] | Hilal, A.A; (2004). New diseases of ornamentals in Egypt: cut flower plant Gardenia. Egyptian J. phytopathol. 32 (1-2), 143-144. |
| [11] | Dimock, A.W. (1940). Epiphytotic of Botrytis blight on Gladiolus in Florida.Pl. dis. Reptr. 8,159-161. |
| [12] | Mullen, J and Jacobi, J. (2001). Botrytis primer. http:// www. Aces. Edu /timely info/ plant pathology/ 2001/ April/ pp 503. PDF |
| [13] | Calvino, E.M. (1939). Two cases of Gardenia canker. Costa azzur.Agric. Flor. 10 (11-12), 189-190. Cited from J. Appl. Mycol. Abstract 231: (1939). |
| [14] | Mc-Kenzie, M.A., Jones, L.H and Gilgut, C.J. (1940). Phomopsis gardeniae in relation to gardenia culture. Pl. Dis. Reptr, 3, 58-62. |
| [15] | Verneau, R. (1949). Contribution to the knowledge of the disease of ornamental plants. Ric. Ossuz. Divulg. Fitopat-companiana-ed mezzogiorno (portico) 11, 1-18. Cited in http:// www. Eul. Edu. Eg. |
| [16] | Buddin, W and Wakefieled, E.M. (1938). Stem canker of gardenias. Gdnrs׳ chron, ciii; 2664, p. 45. |
| [17] | Hansen, H.N and Barrett, J.I. (1938). Gardenia canker. Mycol. 1, 15-19. |
| [18] | Lingk, W. (1991). Health risk evaluation of pesticide contamination in drinking water. Gesunde Pflangen. 43, 21-25. |
| [19] | Daferera, D.J; Ziogas, B.N and Polissiou, M.C. (2003). The effectiveness of plant essential oils on the growth of Botrytis cinerea, Fusarium spp. and Clavibacter michigenensis sub sp. michiganensis. Crop protect.. 22, 39-44. |
| [20] | Tewari, S.N. (1990). Toxic effect of few botanicals on three fungal pathogens of rice. In proc. Symposium Botanical Pestcides in IPM. Eds. Chari, M.S. and Ramprasad, G. pp. 397-403. |
| [21] | Badei, A.Z.M., El-Akel, A.T.M., Morsi, H.H., Baruah, P., Sharma, R.K., Singh, R.S and Ghosh, A. (1996). Fungicidal activity of some naturally occurring essential oils against Fusarium moniliforme. J. Essent. Oil Res. 8, 411-412. |
| [22] | Bishop, C.O and Thornton, I.B. (1997). Evaluation of the antifungal activity of the essential oils of Monarda citriodora var. Citriodora and Melaleuca alternifolia on postharvest pathogens. J. Essent. Oil Res. 9, 77-82. |
| [23] | Tripathi, A.K., Prajapati, V., Verma, N., Bahl, J.R., Bansal, R.P. and Khanuja, S.P.S. (2002). Bioactivities of the leaf essential oil of curcuma longa (var. ch-66) on three species of stored product beetlets (Coleoptera). J. Econ. Entomol. 95, 183-189. |
| [24] | Oxenham, S.K. (2003). Classification of an Ocimum basilicum germplasm collection and examination of the antifungal effects of the essential oil of basil. Ph.D. thesis, Glasgow, UK. Univ. Glasgow. Cited in http:// www. Eul. Edu. Eg. |
| [25] | Wilson, L.C., Solar, Elghoouth, J.M.A, and Wisniewski, M. E., (1997). Rapid evaluation of plant extract and essential oils for antifungal activity against Botrytis cinerea. Pl. Dis. 81 (2) 204-210. |
| [26] | Bhaskara Reddy, M.V., Angers, P., Gosselin, A., Arul, J. (1998). Characterization and use of essential oil from Thymus vulgaris against Botrytis cinerea and Rhizopus stolonifer in strawberry fruits, Phytochem. 47 (8), 1515-1520. |
| [27] | Bakkali, F., Averbeck, S., Averbeck, D., Zhiri, Abaudoux, D., Idaomar. M. (2008). biological effects of essential oils-Areview. Food and chemical toxicology. 46 : 446-475. |
| [28] | Begamboula, C.F., Uyttendaele, M and Debevere, J. (2004). Inhibititory effect of thyme and basil essential oils, carvacrol, thymol, estragol, linalool and p-cymene towards Shigella sonnei and S. flexneri. Food Microbiol. 21, 33-42. |
| [29] | Ghfir, B; Fonvieille, J.L and Dargent, R. (1997). Influence of essential oil of Hyssopus officinalis on the chemical composition of the walls of Aspergillus fumigatus. Mycopathol. 138, 7-12. |
| [30] | DeBillereck, V.G., Roques, C.G; Bessiere, J.M; Fonieille, J.L and Dargent, R.(2001). Effects of Cymbopogon nardus W. Watson essential oil on the growth and morphogenesis of Aspergillus niger. Can. J Microbiol. 47, 9-17. |
| [31] | Knobloch, K; Pauli, A., Iberl, B., Weis, N and Weigand, H. (1989) Antibacterial activity and antifungal properties of essential oil components. J. Essent. Oil Res. 1, 119-128. |
| [32] | Melo, G.A., Shimizu, M.M., Mazzafera, P. (2006). Polyphenoloxidase activity in coffee leaves and its role in resistance against the coffee leaf rust. Phytochem. 67, 277-285. |
| [33] | Farmer, E.E. (2001). Surface to air signales. Nature. 411. 854-856. |
| [34] | Dhingra, O.D and J.B. Sinclair. (1985). Basic plant pathology methods. CRC Press, Inc., Florida, USA, 355 PP. |
| [35] | Ellis, M.B. (1971). Dematiaceoushyphomycetes.Commonwealth mycological institute. Kew, surrey, England. |
| [36] | Raper, K.B. and Fennel, D.I. (1977). The genus Aspergillus. Krieger, R.F. publishing company Huntington, New York. |
| [37] | O’donnel, K.L. (1979). Zygomycetes in culture. Fuller M.S. (Ed.).Georgia Univ., Georgia. |
| [38] | Baudion, A.B. (1988). Laboratory exercises in plantpathology. An Instruction kit. The American psychopathological society. St. paul, Minnesota. |
| [39] | Gulluce, M., Sokmen, M., Deferera, D., Agar, G; Ozcan, H., Kartal, N., Polissiou, M., Sokmen, A and Sahin, F. (2003). The In vitro antibacterial, antifungal and antioxidant activities of the essential oils and menthol extracts of herbal parts and callus culture of Satureja hortensis L. J. Agri. Food Chem. 51 (14), 3958-3965. |
| [40] | Deans, S.G., Svoboda, K.P., (1990). The antimicrobial properties of marjoram (Origanum majorana L.) volatile oil. Favour fragar. J. 5, 187-190. |
| [41] | Zewani,Q. K., Bahadur, P. and Sharma, P. (2004). Effect of fungicides and neem extract on mycelial growth and myceliogenic germination of Sclerotinia sclerotiorum. Indian Phytopathol. 57 (1), 101-103. |
| [42] | Gonsalves, A.K and Ferreira, S.A. (2002). http://www.Extent. Hawaii. Edu/ base/ crop/ type/ bot_prim. Htm. |
| [43] | Kobayashi, T and Nakashima, C. (2002). Addition and re-examination of Japanese species belonging to the genus Cercospora and allied genera. V. collections from nansi islands. Mycosci.43, 219-227. |
| [44] | Maruzzella, J.C. (1962). The germicidal properties of perfume oil and perfumery chemicals. Am. perfumer, 77-67. |
| [45] | Jaspal, S and Tripathi, N.N. (1999). Inhibition of storage fungi of black gram (Vigna mungo L.) by some essential oils. Flavour fragr, J. 14: 1-4. |
| [46] | Aligianis, N; Kalpoutzakiis, E and Chinou, I.B. (2001). Composition and antimicmicrobial activity of the essential oil from origanum species. J.Agri. Food chemist. 49, 4168-4170. |
| [47] | El-Baroty, G.S. (1988). Biochemical studies on some naturally occurring substances and their relation to lipid oxidation. Ph.D. thesis. Fac. Agri., Cairo Univ. |
| [48] | Mussa, F.R. (1998). Biochemical studies on some medicinal and aromatic plants. Food science Department, Ph.D. Thesis. Faculty of agriculture, Moshtohor, Zagazig University. |
| [49] | Mohamed, S.M., Khter, M.R and Moussa- Faten. R (2003). Antifungal activity of some volatile oils towards phytopathogenic fungi. Minufia J. Agric. Res. 28 (4), 1145-1157. |
| [50] | Arras, G and Usai, M. (2001). Fungitoxic activity of 12 essential oils against four postharvest citrus pathogens: Chemical Effect in sub-atmospheric pressure conditions. J. Food Protect. 64 (7), 1025-1029. |
| [51] | Saikaia, D., khanuja, S.P., Kahol, A.P., Gupta, S.C and Kumar, S. (2001). Comparative antifungal activity of essential oils and constituents from three distinct genotypes of Cymbopogon spp. Curr. Sci. 80, 1264-1266. |
| [52] | Boyras, N and Ozcan, M. (2006). Inhibition of phytopathogenic fungi by essential oil, hydrosol, ground material and extract of summer savory (satureja hortensis L.) growing wild in turkey. International J. food microbiol. 107. 238-242. |
| [53] | Anderson, R.A; Hamilton-Kemp, T.R; Hilderbrand, D.F; McCracken, C.T; Collins, R.W and Fleming, P.D. (1994). Structure antifungal activity relationships among volatile C6 and C9 aliphatic aldehydes, ketones and alcohols. J. Agri. Food chemist. 42, 1563-1568. |
| [54] | Feng, W and Zheng, X. (2007). Essential oils to control Alternaria alternata In vitro and In vivo. Food control. 18 (9), 1126-1130. |
| [55] | Kishore, G.K; Pande, S; Harish, S. (2007). Evaluation of essential oils and their components for broad spectrum antifungal activity and control leaf spot and crown rot disease in peanut. Pl. Dis. 41(4), 375-379. |
| [56] | Bakkali, F., Averbeck, S., Averbeck, D., Zhiri, Abaudoux, D., Idaomar. M. (2005). cytotoxicity and gene induction by some essential oils in the yeast saccaromyces cerevisiae. Mutat. Res. 585, 1-13. |
| [57] | Bakkali, F., Averbeck, S., Averbeck, D., Zhiri, Abaudoux, D., Idaomar. M. (2006). antigenotoxic effects of three essential oils in diploid yeast saccharomyces cerivisiae after treatments with UVC radiation, 8- MOP plus UVA and MMS. Mutat. Res. 606, 27-38. |
| [58] | Hansen, J.M., Go. Y.m., Jones, D.P. ( 2006) Nuclear and mitochondrial compartmentation of oxidative stress and redox signaling. Annu. Rev. Pharmacol. Toxicol. 46, 215-910. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML