-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Microbiology Research
p-ISSN: 2166-5885 e-ISSN: 2166-5931
2013; 3(4): 148-151
doi:10.5923/j.microbiology.20130304.04
Studies on Keratinase Producing Fungi Isolated from Poultry Waste and their Enzymatic Activity
Gampa Ramakrishnaiah, S. M. Mustafa, G. Srihari
Department of Biotechnology, Sree Vidyanikethan Engineering College, Tirupati, 517102, A.P. India
Correspondence to: Gampa Ramakrishnaiah, Department of Biotechnology, Sree Vidyanikethan Engineering College, Tirupati, 517102, A.P. India.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
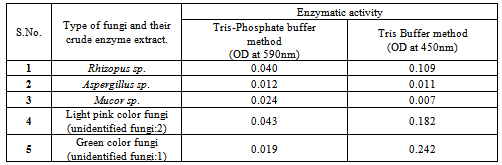
Keratins are the most abundant proteins in epithelial cells of vertebrates and represent the major constituents of skin and its appendages such as nail, hair, feather, and wool. World-wide poultry processing plants are producing millions of tons of feathers as waste products annually, which consist of approximately 90% of keratin. In the present study from the decaying poultry feather samples five types of indigenous fungi were isolated and their enzymatic activity was measured. Each one of these isolated fungi showed variation in their enzymatic activity in different methods. In tris-buffer method, we found the green colored fungi with highest activity and yellow-green colored fungi with least activity. In the tris-phosphate buffer method, we found white colored fungi with highest activity and light pink colored fungi with least activity. From the overall analysis, we found white colored fungi with optimal activity in all the three methods. In these five isolates we identified white colored fungi as Rhizopus spp. black colored fungi as Aspergillus spp. and yellow green colored fungi as Mucor spp.
Keywords: Feathers, Fungi, Keratinase, Poultry Waste
Cite this paper: Gampa Ramakrishnaiah, S. M. Mustafa, G. Srihari, Studies on Keratinase Producing Fungi Isolated from Poultry Waste and their Enzymatic Activity, Journal of Microbiology Research, Vol. 3 No. 4, 2013, pp. 148-151. doi: 10.5923/j.microbiology.20130304.04.
Article Outline
1. Introduction
- Keratin is the major component of poultry feathers waste. High protein content of keratin waste can be used as a good source of protein and amino acids by systemic recycling. Recycling of feather can provides a cheap and alternative protein feed stuff. Natural degradation of poultry feathers was observed in many studies. Mechanical stability and high resistance to proteolytic degradation of keration are due to their disulfide bonds, hydrogen bonds, salt linkages and cross linkings[1]. Keratinase producing microorganisms have the ability to degrade chicken feathers, hair, nails, wool etc[2 & 3]. Recycling of feathers is subject of interest, because it is a potentially cheap and alternative protein supplement to be used as animal feed[4]. Distribution of keratinolytic microbes in the nature is widespread. Some fungal strains can produce keratin proteases which have keratinolytic activity and are capable to keratinolyse feather α-keratin. These enzymes has been produced by fungi, including the species of Aspergillus, Onygena, Absidia and Rhizomucor[5] and some species of dermatophytes, including Trichophyton mentagrophytes, T. rubrum, T. gallinae, Microsporum canis and M. gypseum etc.,[6]. In the present study we screened various poultry waste samples collected from Tirupati urban regions for keratinase producing fungi and their enzymatic activity was evaluated.
2. Materials and Methods
2.1. Sample Collection
- The poultry waste soil samples were collected from various areas of Tirupati urban A.P. India. Samples were collected from 3 to 4 cm depth contained decaying feathers and transferred in sterile plastic bags for further study.
2.2. Isolation of Keratinolytic Fungus
- Isolation of fungi was performed by serial dilution and plating methods on potato dextrose agar medium (PDA). One gram of decaying feather soil sample was transferred in 10 ml of sterilized distilled water and mixed properly. Serial dilution was made up to 10-7. Then 0.1 ml of the diluted samples of different dilutions was inoculated in the PDA plates individually. The Petri plates were rotated clockwise and anticlockwise to spread the sample uniformly. Plates were incubated at 37°C for 5 to 7 days in an inverted position. The fungal isolates were further sub cultured on PDA medium to obtain pure culture. For pure cultures the medium was supplemented with 1% chicken feather powder as sole source of carbon and nitrogen along with g/l 0.5 NaCl, 0.4KH2PO4, 0.5MgSO4, 1.0 K2HPO4, 20.0 Agar.Keratinolytic activity of fungi was detected as clear zones around the colony after incubation for up to 7 days at room temperature. The selected keratinolytic fungi spores were suspended in the distilled water and stored for future use. We isolated five types of fungi that showed keratinolytic activity.
2.3. Identification of Isolated Fungus
- Staining with lacto phenol cotton blue and microscopic observations were performed to identify the isolated fungal cultures.
2.4. Production & Extraction of Enzymes from Isolated Keratinolytic Fungus
- After incubation, 10 ml of culture was transferred to 250 ml medium. The medium was prepared similarly as previously described. All incubations were done at 30℃ with shaking at 150 rpm in a controlled environment shaker. These flasks were incubated for 5-14 days. The purpose of this step was only large scale (i.e. in sufficient amount for biochemical testing) production of keratinase enzyme.
2.5. Filtration
- The culture medium was filtered through Whatman No.1. Filter paper to remove residual non degraded feathers and mycelium. The filtrate was then subjected to centrifugation at 10,000 rpm for 10 min to remove fungal residue. All five different fungal cultures and their enzymes extractions were isolated. Then these crude enzyme isolates were used for enzyme assay.
2.6. Determination of Enzymatic Activity
- Keratinolytic activity of isolated five types of fungal crude enzyme extracts were evaluated spectrophotometrically after incubating Azo-keratin with crude enzyme samples at 50℃ , pH – 7.5 for 15minutes by the following two types of methods: a) Tris-buffer method [7] (Yamamura et al., 2002). b) Tris-Phosphate buffer method[8]. 1) Tris buffer method (0.1M, pH 8.0):20 ml of 0.1M tris buffer (pH 8.0) containing 0.1% feather and 40 µl of enzyme solution and was incubated for 30 minutes at 55℃. The reaction was stopped with 500 µl of 0.1M trichloroacetic acid (TCA) in 0.1 mol tris buffer, pH 8.0. The amino acids liberated were measured as the absorbance at 590 nm against reagent blank. One unit (U/ml) of keratinolytic activity was defined as an increase of corrected absorbance of 590 nm (A 590) with the control for 0.01 per minute.2) Phosphate buffer method (50 mM, pH 7.5.):This procedure was used for the testing of keratinolytic activity of keratinase on azo-keratin. 5 mg of azo-keratin was added to a 1.5-ml centrifuge tube along with 0.8 ml of 50 mM potassium phosphate buffer, pH 7.5. This mixture was agitated till azo-keratin was completely suspended. A 0.2-ml aliquot of supernatant of crude enzyme was added to the azo-keratin, mixed and incubated for 15 min at 50℃ with shaking. The reaction was terminated by adding 0.2 ml of 10% trichloroacetic acid (TCA). The reaction mixture was filtered and its activity was analyzed. The absorbance of the filtrate was measured at 450 nm with a UV-Vis spectrophotometer. A control sample was prepared by adding the TCA to a reaction mixture before the addition of enzyme solution. A unit of keratinase activity was defined as a 0.01 unit increase in the absorbance at 450 nm as compared to the control after 15 min of reaction.
3. Results and Discussion
- From the collected 20 samples, five types of keratinolytic fungi were isolated as pure cultures. The visible appearance of grown fungal mycelia on Petri plates were observed as white color, yellow green color, light pink color, black color and green color fungi. Based on staining and microscopic observations among these five cultures three were identified as Rhizopus sp.[9] (white colored fungi), Aspergillus spp. (black colored) and Mucor species (yellow green colored fungi), the remaining two were not identified. The enzymatic activity of isolated cultures showed variations in different methods. In tris buffer method unidentified fungi (green color culture) showed high enzymatic activity (0.242) followed by unidentified (light pink color) fungi (0.182) and Rhizopus sp.(0.109).In tris-phosphate buffer method all isolates showed less activity, this may be due to the variations in the buffer and in the pH of the solution. These results showed that the type of buffer and its pH plays an important role on the keratinolytic activity of the isolated cultures. Selected species of Aspergillus have been identified as prospective producers of keratinolytic enzymes[10]. The present results showed that Rhizopus sp. and two unidentified fungal species also have high keratinolytic activity. The present study also indicated that the type of buffer system and pH of the culture medium influences the production of keratinases and their activity on keratins degradation.
4. Conclusions
- A number of microorganisms including different fungi are present in the environment and utilizing keratins as their nutrient source. Effective screening and exploitation of these fungi for their keratinolytic enzymes, makes betterment of biodegradation of poultry feathers waste and also production of animal protein feed, which has in huge demand from livestock farms. The present study indicates that the presence of keratinolytic fungi in the poultry waste soils of Tirupati region of Andhra Pradseh and India.
|
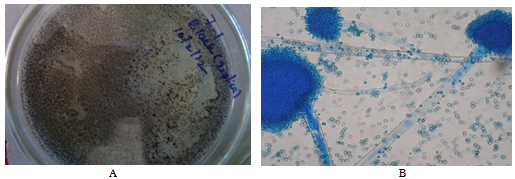 | Figure 1. A: Isolated fungal mycelia appeared in black colour, B: Microscopic image (Aspergillis sp.) |
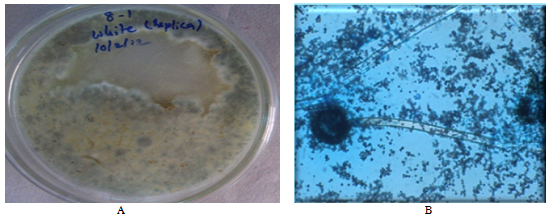 | Figure 2. A: White color fungi B: Microscopic image (Rhizopus sp.) |
 | Figure 3. A: Green color fungi B: Microscopic image (Mucor sp.) |
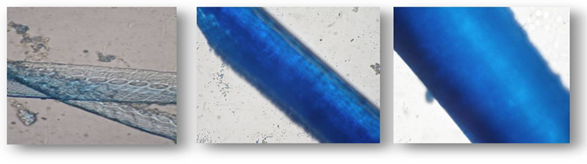 | Figure 4. Stained feathers before degradation (100X magnification) |
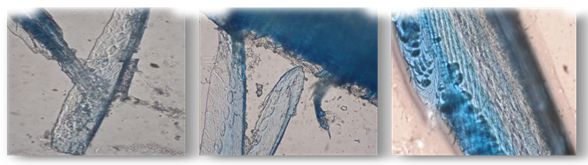 | Figure 5. Stained feathers after degradation (100X magnification) |
ACKNOWLEDGMENTS
- Authors are grateful to the Management of Sree Vidyanikethan Engineering College for their continuous support and providing facilities to carry out the work.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML