-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Microbiology Research
p-ISSN: 2166-5885 e-ISSN: 2166-5931
2013; 3(3): 124-129
doi:10.5923/j.microbiology.20130303.04
Impact of Combination of Lactic Acid Bacteria and Yeasts in Fermentation of Jibna-beida
Abdel Moneim E. Sulieman1, Walied A. Mustafa2, Warda S. Abdelgadir3, Elamin A. Elkhalifa4
1Department of Biology, Faculty of Science, University of Hail, Hail, Kingdom of Saudi Arabia
2Department of Food Science, Faculty of Agriculture, University of Bakht-Elruda, El-Dueim, Sudan
3Food Research Centre, Ministry of Science and Technology, Khartoum, Sudan
4Department of Food Science and Technology, Faculty of Engineering and Technology, University of Gezira, Wad-Medani, Sudan
Correspondence to: Abdel Moneim E. Sulieman, Department of Biology, Faculty of Science, University of Hail, Hail, Kingdom of Saudi Arabia.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Jibna-beida, a semi-traditional cheese of Sudan made from raw cow’s milk, goat’s milk or combination of both with variable qualities. The present study aimed to investigate the impact of using lactic acid bacteria (LAB) and yeasts initially isolated from traditional cheese in production of laboratory made jiba-beida under controlled conditions and evaluation of the product. The activity of LAB isolates was examined via determination of clotting time, presence of inhibitory activity against pathogenic microorganisms and antibiotic resistance. Two types of jibna-beida were produced: LSKJ cheese prodcued using Lactobacillus plantrum, Streptococcus thermophillus and Klaveromyces lactis as combined starter and LSSJ, cheese produced using Lactobacillus lactis, Streptococcus lactis, andSaccharomyces rouxii as combined starter. Significant changes were found in most of the various chemical components of tested cheeses as a result of using different starter cultures. The microbiological analysis revealed absence of colifoms as well as pathogenic bacteria (staphylococci and salmonella) in all tested cheeses. However, 2.85×105± 0.421 cfu/g total bacterial count in were found in LSKJ which were lower than those of LSSJ (6.55 × 105± 2.425 cfu/g. On the other hand, LAB count of LSKJ (7.7×104± 0.462 cfu/g) was lower than that LSSJ (5.6 × 105± 4.041 cfu/g). The yeasts and moulds was 5.0 × 102± 1.155 cfu/g and 4.0 × 103± 4.041 cfu/g LSKJ and LSSJ, respectively. The laboratory made jibna-beida samples were highly acceptable by the panelists. The study demonstrated the potential probiotic ability of the isolated LAB species from jibna-beida prepared at house hold level at El Dueim area.
Keywords: Jibna-beida, Lactic Acid Bacteria, Antimicrobial Activity, Antibiotic Resistance
Cite this paper: Abdel Moneim E. Sulieman, Walied A. Mustafa, Warda S. Abdelgadir, Elamin A. Elkhalifa, Impact of Combination of Lactic Acid Bacteria and Yeasts in Fermentation of Jibna-beida, Journal of Microbiology Research, Vol. 3 No. 3, 2013, pp. 124-129. doi: 10.5923/j.microbiology.20130303.04.
Article Outline
1. Introduction
- Jibna-beida (white cheese) is one of the most cheeses available in Sudanese market. Jibna beida invariably contains lactic acid bacteria (LAB), yeasts and coliform bacteria. In the cheese on the market, yeasts have been found to dominate the microflora[1]. On prolonged storage, however, LAB increased to much higher levels than the yeasts[2].Lactic fermentation has been known for a long time and the expression “Lactic acid bacteria” was used by the early bacteriologists to describe those bacteria that spontaneously soured traditional lactic acid fermented foods.In developed countries, most of the lactic acid fermentation has been concentrated in dairy and vegetable products while in the developing countries and in particularly Africa, lactic acid fermentation predominates all the indigenous processing of cereals like maize, sorghum, millet and root crops such as cassava[3]. Several previously published reports have indicated the presence of Lactobacillus strains in sheep and cow milk[4]. In addition, several studies have shown the inhibitory activities of numbers of LAB such as Lactobacillus brevis isolated from Turkish dairy products[5]. and Lactobacillus acidophilus isolated from Iranian yoghurt against Staphylococcus aureus[4].Antimicrobial activity of lactic acid bacteria isolated from milk products has been the subject of intensive research due to the potential application of these bacteria as productive cultures in biological preservation[6]. The major groups of inhibitory compounds produced by LAB include: lactic acid and other volatile acids (decrease the pH), other primary metabolites such as hydrogen peroxide, carbon dioxide , diacetyl, and bacteriocins- special antimicrobial compounds.Each of these groups of compounds, especially a combination of them, can be used to extend the shelf life and safety of food products. Starter culture is very important for fermentation processes. Growth of fermenting microorganisms can be quite slow for some species under certain conditions when the concentration of cells is too low. Log-phase growth is powerful, and so one would like to keep cells in this state for the experiment at hand. Different genes are expressed then compared to a stationary phase.In addition, for the culture to out-compete a contaminant if there is one. That is more easily accomplished with a starter culture, which is then used to inoculate a larger culture for scale-up. Inoculating directing into the large-sized flask may allow your bacteria to enter a stationary phase, thus giving an opportunity for other species to out-compete your bacteria. The objective of the present study:
2. Materials and Methods
2.1. Bacterial Strains and Culture Conditions
- The LAB strains used in the present study were grown in MRS broth (HiMedia India) at 37ºC for 24-48 h in anaerobic jars. The pathogenic strains used in this study were grown in BHI (HiMedia-India) at 37ºC for 18-24 h under aerobic condition. All strains were maintained at 4˚C (in aerobic condition). The purified isolates were preserved in MRS broth with sterile glycerol (15%) and stored at -70ºC (Badis et al., 2004). The isolated LAB were tentatively identified as Lactobacillus plantrum, Lactobacillus lactis, Streptococcus thermophillus and Streptococcus lactis.
2.2. Activation Test of LAB
- To determinee the activity of the LAB isolated from collected cheese samples to be used in production of laboratory made jiban-beida, the following tests were carried out:
2.3. Clotting times and Acidification Rates
- To determinee the clotting times and acidification rates of LAB, equal volumes of milk (10 ml) were placed in four test tubes, which were incubated with about (108 cfu) of Lactobacillus plantrum, Lactobacillus lactis, Streptococcus thermophillus and Streptococcus lacti. The clotting times and acidification rates were recorded after 1- 8 hours.
2.4. Antimicrobial Activity Against Pathogens
- Twenty eight LAB isolates demonstrating high antibacterial activity against other selected LAB isolates were further checked against other Gram positive and negative pathogens by agar well diffusion method, the bacterial strains which were used as indicator culture included: Salmonella spp, Pesudomonas aeuriginosa, Staphylococcus aureus and E. coli O157:H7 were used as test strains to evaluate the antimicrobial effects of LAB isolated from cheese. Salmonella sp., Pesudomonas aeuriginosa and Staphylococcus aureus isolates were obtained from the Food Research Center and from Faculty of Animal Production, Khartoum University. E. coli O157:H7 was isolated from hamburger meat and was obtained from the Food Microbiology Laboratory of Khartoum University. The antimicrobial activities of the isolates were quantified by modifying the disc diffusion. A well-isolated colony was selected from MRS agar plate culture. The top of the colony was touched with a loop, and the growth was transferred into a tube containing sterile 5ml MRS broth. Then broth culture was incubated at 37 ̊C for about 24 hrs. To get the culture filtrate a 24hrs cultures were centrifuged (10,000 rpm for 20min, at 4 ̊C) then was adjusted to pH 7 by 1M NaOH to exclude antimicrobial effect of organic acid[7]. Two control test materials also were prepared using un-inoculated MRS broth.An actively growing test (indicator) microorganisms in a Tryptone Soya broth (OXOID of 24hrs culture at 35 ̊C were dipped with a sterile cotton swab which was rotated several times and pressed firmly on the inside wall of the tube above the fluid level to remove excess inoculum from the swab. The dried surface of a Mueller-Hinton agar (HIMIDIA) plates were inoculated by streaking the swab over the entire sterile agar surface. This procedure was repeated by streaking two more times, rotating the plate approximately 60 ̊C each time to ensure an even distribution of inoculum. A sterile approximately 6 mm in diameter discs (Whatman filter paper No. 1) were delivered with culture filtrate of each isolate by using a wire loop of 20 gauge wire having 2mm of diameter, that can deliver 5μl of the extract to each disc[8].Four discs (with culture filtrate) were placed on a 100mm plate within 5 to 15 minutes of striking of the test organisms. After 12 and 24 hours of anaerobic incubation, each plate was examined. The diameter of the zones of complete inhibition was measured, including the diameter of the disc. Zones are measured to the nearest whole number in millimeter, using transparent ruler. Method assay procedure of Tadese et. al.,[9] was used.
2.5. Antibiotic Resistance
- Four strain identified L. lactis, L. thermophillus, S. Lactis and S. plantarum, were selected on the basis of their desirable technological characteristics[10]. They were tested for resistance to 8 antibiotics produced by Biodiscs were; Chloramphenicol (25mcg), Erythromycin (5mcg), Fusidic acid (10mcg), Methicllin (10mcg), Novobiocin (5mcg), Penicillin-G (1unit), Streptomycin (10mcg), Tetracycline (25mcg). This testing was performed using the standard disc diffusion method .
2.6. Manufacture of Jibna-beida
- Jibna-beida was made as described by Osman[11] using two types of starter cultures, (1) starter culture consisting of Lactobacillus plantrum, Streptococcus thermophillus and Klaveromyces lactis as combined starter (LSK), and (2) starter culture consisting of Lactobacillus lactis, Streptococcus lactis, and Saccharomyces rouxii as combined starter (LSS). Those LAB and yeasts strains (2%) which were previously isolated from collected cheese samples, were added to the pasteurized milk at 40 ºC, then rennet (0.07 % g/L) was dissolved in milk. Salt (7 g/L) and the inoculated milk were stirred for 5 minutes, and then the mixture was left to develope a curd. The curd after coagulation was cut by stainless kitchen knife and left for 5 minutes to separate the whey. This was collected, and kept in room temperature. The curd was collected and transferred into clean wooden moulds lined with clean clothes then pressed with 1 kg weight overnight. In the next day the curd was cut into cubes and weighted, then transferred into plastic containers and the whey was added. Cheese was then stored for 1 day first, and then transferred to refrigerator at 5 ºC.
2.7. Chemical Analysis of Laboratory-made Jibna-beida
- The various chemical analysis which included mositure, protein, fat, ash, total soluble solid,, lactose and pH were determined in laborotary- made jibna-beida according to AOAC[12].
2.8. Microbiological Analysis of Laboratory-made Jibna-beida
- The various microbiological analysis which included total bacterial count, coliform, Staphylococcus aureus, yeast and mould, detection of Salmonella and lactic acid bacteria were estimated in laborotary- made jibna-beida using standard methods.
2.9. Sensory Evalution
- Jibna-beida sample were subjected to sensory evalution using (15-20) panelists, the panelists were asked to assess each sample for colour, appearance, flavour, texture and overall acceptability A 9 point hedonic scale with 1 as the extremely bad and 9 the excellent. All analysis took place in a room free from disturbing noises, and in which fresh air was circulation conditions were equalized for all the tests. The order of presentation for samples was randomized and the samples were given codes before being tested.
2.10. Statistical Analysis
- Statistical analysis was done using Statistical Package for Social Studies Software[13]. Complete randomized design was used to estimate chemical, microbiological and sensory characteristics of the jibna-beida.
3. Results and Discussion
3.1. Activation test of LAB using for Manufacture of Jibna-beida
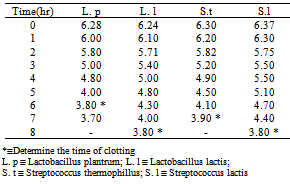
- Table (1) shows the time/hour of clotting and acidification rates of Lactobacillus plantrum, Lactobacillus. lactis, Streptococcus. thermophillus and Streptococcus. lactis, the result shows that the time of clotting rates of these microorganisms were after 6, 8, 7 and 8 hours, respectively. These results indicate that the Lactobacillus plantrum was faster to clot ( after 6 hours). On the other hand the acidification rates according to clotting time were 3.8, 3.8, 3.9 and 3.8, respectively.
|
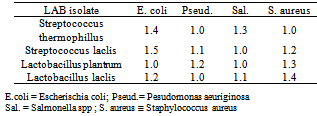
3.2. Antimicrobial Activity
|
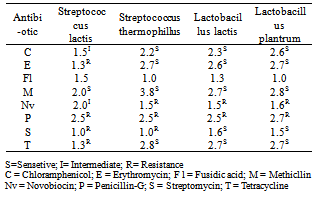
3.3. Antibiotic Resistance
- Table (3) shows the antibiotic resistance/hour of LAB. The result shows that Streptococcus lactis was moderately sensitive to Chloramphenicol and Novobiocin antibiotic; resistant to erythromycin, penicillin-G, streptomycin and tetracycline antibiotic; sensitive to Methicillin antibiotic. On the other hand Streptococcus thermophillus was resistant to Novobiocin, Penicillin-G, and Streptomycin antibiotic; sensitive to Chloramphenicol, erythromycin, Methicllin and Tetracycline antibiotic. Lactobacillus. Lactis resistant to Novobiocin and Penicillin-G antibiotic; sensitive to Chloramphenicol, erythromycin, Methicillin, Streptomycine and Tetracycline antibiotics. Generally most of the LAB isolated from collected cheese samples are resistant, that indicating their high activity during fermentation.
|
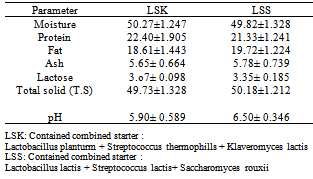
3.4. Chemical Composition of Laboratory Made Jibna-beida
- The chemical composition of laboratory – made jibna-beida is presented in Table (4). The moisture content of jibna-beida produced by using combined starter LSK (LSKJ) (50.27±1.25%) was higher than that produced by using combined starter LSS (LSSJ) (49.82±1.33%). However, these findings were higher than those reported by Aly and Galal[14], Sulieman[15] and Ibrahim[1] who found that the moisture contents in pasteurized milk jibna-beida were 47.28, 43.0, and 44%, respectively.The proteins content of LSKJ (22.40±1.905%) was higher than that LSSJ which was 21.33±1.241%. These values were higher than that determined by Salih[16], who found average of (19.84%) in white cheese made from pasteurized milk. Fat content of LSKJ (18.61±1.443) was slightly lower when compared with that of LSSJ which was 19.72±1.22%. These results were slightly lower than that reported by Salih[16] in jibna-beida produced from pasteurized milk and Elowni and Hamid[17] in Sudanese white cheese, who found a value of 20.30 and 22.8%, respectively . Table (4) also shows that the range of ash content was 5.65±0.664% and 5.78±0.739% for cheese produced from combined starter culture LSK and LSS, respectively. While the lactose content was 3.07±0.098% and 3.35±0.185% in LSKJ and LSSJ, respectively. The change in lactose and protein of jibna beida produced by using different starter culture might be due to microbial enzymatic action, normally the chemical changes of carbohydrate appear in conversion of lactose to lactic acid and small amount of acetic acid and pyruvic acid in addition to CO2. As for fats, they are hydrolyzed to glycerol and fatty acids as a result of microbial action[18].
|
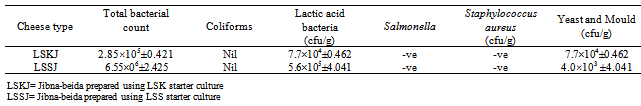
3.5. Microbiological Characteristics of Laboratory Made Jibna-beida
- The microbiological analysis of laboratory made jibna– beida (Table 5 ) revealed presence of 2.85×105± 0.421 cfu/g of total bacterial count in jibna-beida made by using LSK starter culture (LSKJ) which were lower than those of laboratory made jibna-beida from LSS starter culture (LSSJ) (6.55 × 105± 2.425 cfu/g), and these results were in close agreement to those reported by Ahmed, (2010), who obtained a value of 2.8×106 cfu/g in cheese manufactured from raw milk and 1.3 ×105 cfu/g in cheese manufactured from pasteurized milk. The lower level of total bacterial count in laboratory made jibna- beida were probably due to the effect of starter culture (LAB) and heat treatment which suppress the growth of microorganisms, and also could be attributed to the proper hygienic condition of cheese production.
|
- LAB count of LSKJ (7.7×104± 0.462 cfu/g) was lower than that LSSJ (5.6 × 105± 4.041 cfu/g). The LAB count in this study was higher than that reported by Hamid and Elowni[21] who reported (5.06 log cfu/g) in Sudanese white cheese. Table (5) also, shows that yeasts and moulds of laboratory LSKJ was lower (5.0 × 102± 1.155 cfu/g) than that of LSSJ (4.0 × 103± 4.041 cfu/g). The result was in close agreement to those reported by Kameni et.al., (2006) and Ahmed, (2010), who report the values 7.6 × 103 cfu/g and 7.0 × 103 cfu/g, respectively. However, the reduction of yeast and mould count could be attributed to the proper hygienic condition employed in cheese. Fortunately, colifoms and the pathogenic microorganism Salmonella and staphylococcus aureus cells were not found in all laboratory made jibna-beida.
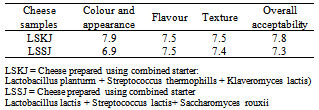
3.6. Sensory Characteristics of Laboratory-Made Jibna-Beida
- Data presented in Table (6) show the mean scores of the sensory characteristics of lab-made jibna-beida made from pasteurized milk and defined starter cultures (LSK and LSS). Comments given by the panelists showed preference for the product which has good texture , colour and appearance, little salty and mild acidity flavour.
|
4. Conclusions
- Jibna-Beida can be prepared in the laboratory under controlled conditions using combinations of dominating LAB (with resistance to antibiotics) and yeasts as starter culture and pasteurized milk. Most of the chemical components of laboratory made-jibna- beida samples were in close agreement with data in the literature. The microbiological analysis revealed absence of colifom bacterial and pathogens (salmonella and staphylococci). The sensory analysis indicated high acceptance of the cheese samples by panelists. The inhibitory activity possessed by LAB isolates might be used for the control of pathogens and spoilage bacteria in jibna-beida and other dairy products especially those produced traditionally. So, the findings of the present study suggest that the selected LAB strains isolated might be appear to possess probiotic potential against pathogens and spoilage microorganisms in traditionally fermented dairy products. Since the selected LAB strains isolated from traditionally made jiban-beida possess probiotic potential, so, they could be exploited further for their use in other fermented dairy products. It is recommended that these LAB species can be further studied according to selection criteria like stimulation of immunological system and adhesion to epithelium tissue.
ACKNOWLEDGEMENTS
- The authors express their gratitude for all who assisted in the implementation of this work, with special thanks to the microbiological laboratory technicians of the University of Gezira.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML