-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Microbiology Research
p-ISSN: 2166-5885 e-ISSN: 2166-5931
2013; 3(3): 117-123
doi:10.5923/j.microbiology.20130303.03
ACC Deaminase Containing Rhizobacteria Enhance Nodulation and Plant Growth in Clusterbean (Cyamopsis tetragonoloba L.)
Aakaknsha Khandelwal, S. S. Sindhu
Department of Microbiology, CCS Haryana Agricultural University, Hisar, 125004, India
Correspondence to: S. S. Sindhu, Department of Microbiology, CCS Haryana Agricultural University, Hisar, 125004, India.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Nodulation on clusterbean (Cyamopsis tetragonoloba L. Taub.) is usually poor leading to low productivity of this commercially important crop. Recently, ACC (1-aminocyclopropane-1-carboxylate) utilizing rhizobacteria have been found to lower the level of nodulation inhibitor ethylene leading to improved nodulation and plant growth of legumes. In this study, Pseudomonas isolates obtained from the rhizosphere of clusterbean were screened for utilization of ACC. Only 38.9% Pseudomonas isolates showed growth on ACC supplemented plates. Inoculation of clusterbean with ACC+ Bradyrhizobium isolate GSA11 or Rhizobium isolate GSA6 formed 45 and 40 nodules, respectively at 60 days of growth under sterilized chillum jar conditions. The coinoculation of ACC+ Pseudomonas isolate CPA123 with ACC+ Bradyrhizobium isolate GSA11 caused 220.7% increase in plant dry weight in comparison to control uninoculated plants at 60 days of growth. In comparison to coinoculation with ACC– Pseudomonas isolate CPA152, the coinoculation of Pseudomonas isolate CPA123 showed more enhancements in symbiotic parameters of clusterbean. Thus, ACC+ rhizobacterial cultures could act as better inoculants for enhancing nodulation and plant growth of clusterbean under field conditions.
Keywords: Clusterbean, ACC utilization, Pseudomonas, Bradyrhizobium, Rhizobium, Coinoculation, Nodulation, Plant Growth
Cite this paper: Aakaknsha Khandelwal, S. S. Sindhu, ACC Deaminase Containing Rhizobacteria Enhance Nodulation and Plant Growth in Clusterbean (Cyamopsis tetragonoloba L.), Journal of Microbiology Research, Vol. 3 No. 3, 2013, pp. 117-123. doi: 10.5923/j.microbiology.20130303.03.
Article Outline
1. Introduction
- In sustainable agriculture, different soil microorganisms with beneficial characteristics are used as bioinoculants to improve productivity of cereal and legume crops, while minimizing the application of chemical fertilizers[1, 2]. Among bioinoculants, nitrogen-fixing microorganisms offer an ecofriendly alternative to nitrogenous fertilizers in farming practices and supply fixed N to crop plants resulting in improved crop production[3, 4]. Some plant growth-growth promoting rhizobacteria (PGPR) promote growth of cereals and legumes by solubilizing bound phosphorus[5] and potassium[6, 7], and by release of vitamins, auxins and plant growth regulating substances[8, 9]. PGPR have also been found to suppress plant diseases caused by potential pathogens by production of antibiotics, siderophores, hydrocyanic acid and/or hydrolytic enzymes[10, 11]. ACC (1-aminocyclopropane-1-carboxylate) present in the root exudates is converted to ethylene (stress hormone) by the enzyme ACC oxidase[12] and ethylene affects plant development at seed germination, morphogenesis, flowering induction and fruit ripening. Ethylene also inhibits root elongation, lateral root growth and root hair formation[13]. During nodulation process, invasion of legume roots by the microsymbiont Rhizobium imposes biotic stress and increases the ACC content of infected roots resulting in production of more ethylene[14]. Thus, ethylene or ethylene precursors (ethephon and ACC) have been reported to inhibit nodulation in Medicago sativa, Pisum sativum and mungbean[15-17]. These biotic or abiotic stresses imposed on the plant are reduced by expression of an enzyme ACC deaminase by some rhizosphere bacteria, which decreases the level of ethylene in host plants[18]. Moreover, chemicals such as L-α-(aminoethoxyvinyl)-glycine (AVG), aminooxyacetic acid (AOA) and rhizobitoxine have also been employed to lower ethylene levels in plants leading to enhancement of nodulation in pea, Lotus japonicus, Macroptilium atropurpureum and Phaseolus vulgaris[19-21]. Rhizobial strains that produce active ACC deaminase, contained a relatively low level of ethylene activity (approximately 2-8%) compared with the amount of enzyme activity generally found in free-living soil bacteria[22, 23]. As a consequence of reduction in ethylene synthesis, nodulation by rhizobia on legumes was enhanced in alfalfa and Lotus japonicus[19], peas[22, 24], chickpea [25] and Medicago truncatula[26]. Earlier, coinoculation studies of PGPR with Rhizobium/Bradyrhizobium spp. have resulted in improving nodulation, root and shoot weight, plant vigor and grain yield of various legumes[27, 28]. Therefore, the hypothesis of this study is that selection of ACC deaminase-containing rhizosphere bacteria having synergistic interactions with Rhizobium/Bradyrhizobium may result in growth-promoting effects leading to improved legume productivity. Clusterbean (Cyamopsis tetragonoloba L. Taub.) is a drought tolerant crop and it is grown during the summer season in the northern arid zone of India. Clusterbean is a rich source of high quality galactomannan gum which is in great demand in the world market because of it multi-purpose use in textiles, foods, cosmetics, mining, explosives and oil industries. However, nodulation status of this crop is poor and only 5 to 10 nodules are formed per plant by native strains[29, 30]. Despite multipurpose use of clusterbean, no systematic work has been done to improve the nodulation, nitrogen-fixing ability and crop productivity using bioinoculants. Thus, it is desired that efficient Rhizobium/Bradyrhizobium cultures should be isolated and introduced in clusterbean growing areas to improve its nodulation status, seed quality and crop productivity[31].
2. Materials and Methods
2.1. Isolation of Pseudomonas sp. from the Rhizosphere Soil
- The rhizosphere soil samples were collected from three different locations of clusterbean grown in Chaudhary Charan Singh (CCS) Haryana Agricultural University, Hisar farm at 30 and 45 days of plant growth. From each location, samples were collected from six different sites and composite rhizosphere soil samples were prepared. The serial dilutions of the rhizosphere soil samples were plated on King’s B medium plates[32]. The plates were incubated at 28±2°C for 3-4 days and rhizobacterial colonies were selected based on morphological and pigment production characteristics. Rhizobacterial isolates were also screened for oxidase test, catalase test, spore staining and Gram staining[33] and Pseudomonas sp. were transferred on Luria Bertani (LB) agar medium slopes[34]. The rhizobacterial isolates were maintained by periodic transfer on LB agar slants and stored at 4°C in refrigerator for further use. ACC utilizing Bradyrhizobium isolate GSA11 and Rhizobium isolate GSA6 along with ACC non-utilizing Bradyrhizobium isolate GSA114 and Rhizobium isolate GSA115 were obtained from the Department of Microbiology[35]. For seed inoculation purpose, seeds of clusterbean variety HG563 were obtained from Department of Seed Science and Technology, CCS Haryana Agricultural University, Hisar.
2.2. Screening of Pseudomonas Isolates for Utilization of ACC
- The medium plates were prepared with minimal medium[36] supplemented with ammonium sulphate (2g L-1) or 3 mM ACC[37]. A loopful of 48-hour old growth of Pseudomonas culture was spotted on the medium plates and incubated at 28±2°C for 2-5 days. The diameter of growth of different bacterial isolates on ACC supplemented medium plates was recorded. The cultures showing good growth on ACC supplemented medium plates and capable of utilizing ACC as nitrogen source, were scored as ACC+.
2.3. Coinoculation Effect of Rhizobacterial Isolates on Nodulation and Plant Growth
- Seeds of clusterbean were surface sterilized with acidic alcohol (concentrated sulphuric acid: ethanol, 7:3) for 3 min and washed thoroughly with several changes of sterilized water. ACC utilizing or ACC non-utilizing Bradyrhizobium/Rhizobium strains were grown in yeast extract mannitol agar medium broth[38] for 3-5 days and Pseudomonas cultures were grown in Luria Bertani (LB) medium broth for 2 days. Growth of each bacterial culture was harvested in 5 ml sterilized water (107-108 cells ml-1 of growth suspension). Surface sterilized seeds of clusterbean variety HG563 were inoculated with 10 ml of selected ACC utilizing and ACC non-utilizing Bradyrhizobium/Rhizobium and Pseudomonas cultures individually or in combination with Pseudomonas (obtained from mixing of 5 ml growth of each bacteria). Inoculated seeds were germinated in sterilized chillum jar assemblies (with three replications for each treatment) containing Sloger’s nitrogen-free mineral salt solution[39] in the lower assembly. Uninoculated seeds were sown as control. After germination, three healthy seedlings were kept in each chillum jar. Quarter-strength Sloger’s nitrogen-free mineral salt solution was used for watering as and when required. The plants were harvested at 30 and 60 days of growth and observations were taken for nodule number, nodule fresh weight, plant dry weight and plant nitrogen. After washing with tap water, nodules were detached from the roots and dried in the folds of filter paper. The nodules were counted and weighed. Shoot portions of the plants were dried in oven at 90°C for 24 h and weighed. Total N content of clusterbean plants was determined colorimetrically using Nessler’s reagent[40]. The absorbance of the samples was read at 400 nm against a reagent blank using a Beckman DU-40 spectrophotometer. Dehydrated NH4Cl was used as a standard.
2.4. Statistical Analysis
- Completely randomized design (CRD) was used for experimental data analysis. All determinations were carried out in triplicate and data presented are average values of three replications. SEM (standard error of means) values were calculated to determine the significant differences between treatment means (±SD).
3. Results
- Invasion of legume roots by Rhizobium during nodule formation exerts biotic stress and increases the ACC content of the infected roots resulting in enhanced ethylene production in alfalfa[14, 17]. This increased ethylene levels probably could be one of the factors for poor nodulation of clusterbean under field conditions.
3.1. Isolation and Screening of Rhizobacterial Isolates for ACC Utilization
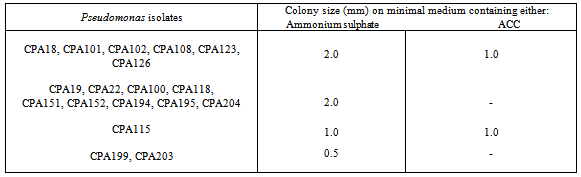
- Eighteen Pseudomonas isolates obtained from the clusterbean rhizosphere were tested for ACC utilization ability on Dworkin and Foster minimal medium plates. Pseudomonas isolates i.e., CPA18, CPA101, CPA102, CPA108, CPA123 and CPA126 showed good growth on ammonium sulphate containing plate and as well as on ACC supplemented plate after 3 days of incubation (Table 1; Fig. 1). Only 38.9% Pseudomonas isolates could grow on ACC incorporated plates. Other isolates CPA19, CPA22, CPA100, CPA118, CPA151, CPA152, CPA194, CPA195 and CPA204 showed good growth on ammonium sulphate containing plate but did not grow on ACC supplemented plate. ACC utilizing Pseudomonas isolate CPA123 and ACC non-utilizing isolate CPA152 were finally selected for coinoculation studies on clusterbean variety HG563 in sterilized chillum jar assemblies.
|
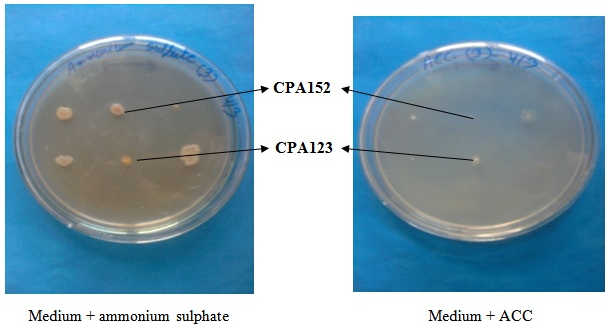 | Figure 1. Growth of Pseudomonas isolates on Dworkin and Foster minimal medium |
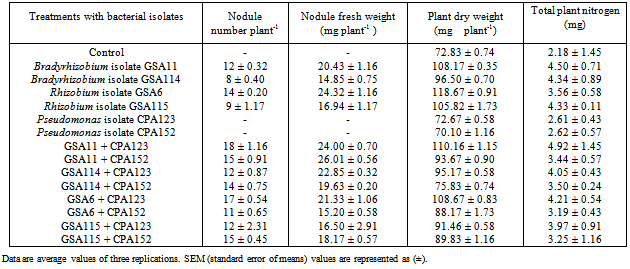
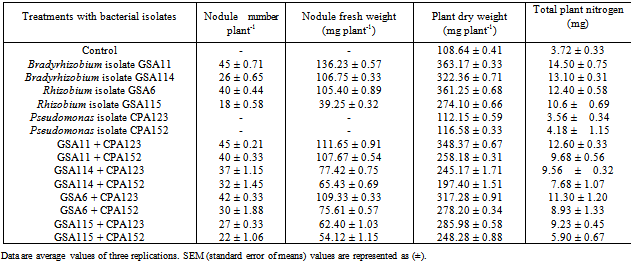
- At 30 days of plant growth, Bradyrhizobium isolate GSA11 formed 12 nodules per plant, and increased plant dry weight by 48.5% as compared to control uninoculated plants (Table 2). Rhizobium isolates GSA6 and GSA115 produced 14 nodules per plant, and plant dry weight was increased by 62.9%. Bradyrhizobium isolate GSA11 when coinoculated with ACC+ Pseudomonas isolate CPA123 produced maximum 18 nodules per plant and increased plant dry weight by 51.3% whereas Rhizobium isolate GSA6 coinoculated with Pseudomonas isolate CPA123 formed 17 nodules per plant and increased only 49.2% plant dry weight as compared to control uninoculated plants. Coinoculation of Rhizobium isolate GSA115 with ACC– Pseudomonas isolate CPA152 produced 15 nodules per plant and increased plant dry weight by 23.3%. All the treatments increased total nitrogen varying from 2.18-4.92 mg. At later stages of plant growth (60 days), ACC+ Bradyrhizobium isolate GSA11 produced maximum 45 nodules per plant and 234.3% increase in plant dry weight was observed, whereas ACC–Bradyrhizobium isolate GSA114 produced only 26 nodules per plant and increased plant dry weight by 196.7% as compared to uninoculated control plants (Table 3). However, when Bradyrhizobium isolate GSA11 was coinoculated with Pseudomonas isolate CPA123, 45 nodules per plant were produced and 220.7% increase in plant dry weight was observed. Similarly, ACC+ Rhizobium isolate GSA6 when coinoculated with Pseudomonas isolate CPA123 produced 42 nodules per plant and plant dry weight was increased by 192.0% in comparison to control uninoculated plants. In different treatments, total nitrogen content also increased from 3.72 mg to 14.50 mg. Thus, coinoculation of ACC+ Pseudomonas isolates with Bradyrhizobium/Rhizobium isolates showed stimulatory effect on nodulation at different stages of plant growth.
|
|
4. Discussion
- Plant rhizosphere is a dynamic environment and preferential niche consisting of heterogenous microbial populations in the soil[41, 42]. Beneficial root associative bacteria including fluorescent pseudomonads and bacilli comprise the major group among PGPR in the rhizosphere. Govindasamy et al.[43] used the utilization of ACC as a sole nitrogen source for growth of rhizobacteria as criterion to select the isolates possessing ACC deaminase activity. In this study, only 38.9% Pseudomonas isolates showed good growth on ACC supplemented plates (Table 1). Similar high frequency of ACC utilization was reported by Husen et al.[44] in which 11 Pseudomonas isolates (out of total 13 isolates), obtained from the soybean rhizosphere, were found to possess ACC deaminase activity. Ma et al.[18] also reported that 38.76% rhizobial strains possess ACC deaminase enzyme. Similarly, 40.0% plant-associated Burkholderia species exhibited ACC deaminase activity[45]. In contrast to above observations, low frequency of ACC utilization was reported in different rhizobacterial isolates and it varied from 3.86% in peanut rhizosphere[46], 10.91% among bacterial isolates obtained from 35 different soil samples[47] and only 11.59% among rhizobial strains[48]. Similarly, screening of 563 bacteria isolated from the roots of pea, lentil and chickpea showed that only 5% isolates showed ACC deaminase activity[49]. Recently, Rashid et al.[50] reported that only 13% of the 174 bacterial endophytes obtained from tomato plant tissues possessed ACC deaminase activity.Biological nitrogen fixation plays a dominant role in sustainable agricultural production[3] and a variety of N2-fixing bacteria have been used to improve the supply of fixed N as nutrient to leguminous and nonleguminous crop plants. However, nodulation of clusterbean by different Rhizobium/Bradyrhizobium strains is usually poor[30]. The number of nodules ranged between 0.93-5.73 in 32 different cultivars of clusterbean (guar), maximum being in cultivar HFG 197[51]. In this study, Bradyrhizobium isolate GSA11 and Rhizobium isolate GSA6 caused 48.5 and 62.9% increase in plant dry weight as compared to uninoculated plants at 30 days of plant growth (Table 2). At 60 days of plant growth, maximum 45 nodules were formed by inoculation of Bradyrhizobium isolate GSA11 and 234.3% increase in plant dry weight was observed, whereas ACC–Bradyrhizobium isolate GSA114 formed only 26 nodules per plant and increased plant dry weight only by 196.7% in comparison to uninoculated control plants (Table 3). Large number of nodules formed by ACC+ rhizobacterial isolates indicated that these ACC deaminase containing rhizobacteria might have lowered the ethylene concentration below the inhibitory level for nodulation of clusterbean. Interestingly, coinoculation of ACC+ Pseudomonas isolate CPA123 with either Bradyrhizobium isolate GSA11 or Rhizobium isolate GSA6 showed better nodulation and plant growth as compared to inoculation with ACC– Pseudomonas isolate CPA152. Thus, these results are potentially important for inoculation purposes of different agricultural crops and bacterial inoculants for different host legumes should first be selected/ tested for the presence of a functional ACC deaminase. In earlier studies, similar synergistic effects have been observed on nodulation and plant growth of other legumes by coinoculation of B. japonicum and P. fluorescens in soybean[27, 52], R. leguminosarum with an antibiotic-producing P. fluorescens strain F113 in pea[53] and Bradyrhizobium/Mesorhizobium strains with Pseudomonas sp. in green gram and chickpea[28, 54]. Dey et al.[46] reported that inoculation of peanut with fluorescent pseudomonad isolates viz. PGPR1, PGPR2 and PGPR4 containing ACC deaminase activity significantly enhanced the pod yield (23-26, 24-28 and 18-24%, respectively), haulm yield and nodule dry weight over the control in field trials. Similarly, a significant correlation was found between in vitro ACC deaminase activity and growth promoting activity of the rhizobacteria on maize under axenic conditions and on nodulation of mungbean (Vigna radiata L.) under natural pot and field trials[55]. These results suggested that inoculation with ACC deaminase containing rhizobacteria augments nodule formation by lowering the level of inhibitory ethylene leading to improved nodulation and plant growth of clusterbean. Therefore, ACC+ rhizobacteria should be extensively screened from the rhizosphere of different crops for improving crop productivity under field conditions.
5. Conclusions
- Rhizobacterial isolates having ACC utilization ability were obtained from the rhizosphere of clusterbean. The coinoculation of ACC+ Pseudomonas isolate CPA123 showed enhanced nodulation and plant dry weights in comparison to coinoculation with ACC– Pseudomonas isolate CPA152 under sterilized chillum jar conditions, suggesting that ACC utilizing rhizobacterial isolates could be exploited as bioinoculants for improving nodulation and plant growth of clusterbean.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML