-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Microbiology Research
p-ISSN: 2166-5885 e-ISSN: 2166-5931
2013; 3(2): 99-105
doi:10.5923/j.microbiology.20130302.07
In Vitro Activity of Ceftazidime and Meropenem in Combination with Tobramycin or Ciprofloxacin in A Clone of Multiresistant Pseudomonas Aeruginosa
Segura C1, Plasencia V1, Ventura E1, Miró E2, Navarro F2, Grau S3, Fusté E4, Montero M5, Horcajada JP5, Viñas M4
1Reference Laboratory of Catalonia
2Microbiology Service. Hospital de la Santa Creu i Sant Pau. Autonomous University of Barcelona
3Pharmaceutical Department. Hospital Universitari del Mar. Autonomous University of Barcelona
4Department of Pathology and Experimental Therapeutics. IDIBELL. University of Barcelona
5Internal Medicine Department. Infectious Diseases Division. Hospital Universitari del Mar. Barcelona. Spain
Correspondence to: Segura C, Reference Laboratory of Catalonia.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Therapeutic options to fight against infections caused by multiresistant (MR) Pseudomonas aeruginosa strains are restricted to a few antimicrobials such as colistimethate and amikacin. The purpose of this study was to compare in vitro synergy testing by epsilometric (E-test) and the checkerboard (CB) methods with time-kill analysis in MR P. aeruginosa clinical isolates. Four isolates belonging to a MR endemic clone were selected. Their resistance mechanisms were studied. Susceptibility to ceftazidime (CAZ) and meropenem (MEM) in combination with tobramycin (TOB) and ciprofloxacin (CIP) were tested. Synergy was consistently detected in CAZ plus TOB as well as in MEM plus TOB combinations. The E-test method was comparable to CB method. Synergy and bactericidal activity were observed at 1/4 or 1/8 MIC TOB concentration combined with 1 MIC of CAZ or 1 MIC of MEM by time kill curves, with slight differences in the two isolates tested. These findings indicate the possibility of designing therapies based on combinations of a β-lactam and an aminoglycoside as a therapeutic option in infections caused by MR P. aeruginosa.
Keywords: Pseudomonas aeruginosa, Synergy, Time-kill, FICI, SPBI
Cite this paper: Segura C, Plasencia V, Ventura E, Miró E, Navarro F, Grau S, Fusté E, Montero M, Horcajada JP, Viñas M, In Vitro Activity of Ceftazidime and Meropenem in Combination with Tobramycin or Ciprofloxacin in A Clone of Multiresistant Pseudomonas Aeruginosa, Journal of Microbiology Research, Vol. 3 No. 2, 2013, pp. 99-105. doi: 10.5923/j.microbiology.20130302.07.
1. Introduction
- Pseudomonas aeruginosa is naturally resistant to a wide variety of antimicrobials and can easily become resistant to many more; this constitutes a serious and growing therapeutic challenge, particularly in hospital setting. In addition, the natural capacity of P. aeruginosa to survive in adverse conditions and their minimal nutritional requirements, as well as their cosmopolitan distribution, enables this bacterium to be a silent nosocomial inhabitant. A large number of publications have pointed out the increasing resistance rates, with special concern on the increase of isolation of multiresistant and panresistant strains[1, 2, 3]. As a result, endemic situations with variable incidences have emerged in many hospitals[4, 5, 6]. The lack of pipeline antipseudomonal agents available make the situation worse in the short-term.This has enhanced the interest in exploring the use of associations of several antibiotics and also in the rescue of old antimicrobials whose use had ceased several decades ago because of its potential toxicity; some of them have been used and assayed in several antibacterial combinations to achieve synergy[7]. The aim of this study was to assess synergistic effect of several antibiotic combinations as well as to explore bactericidal effect on P. aeruginosa isolates belonging to this endemic clone, thus, we explored the combinations of ceftazidime and meropenem with tobramycin and ciprofloxacin in four representative multiresistant P. aeruginosa isolates belonging to a nosocomial endemic clone of the Hospital del Mar, in Barcelona. Preliminary experiments to explore the mechanisms of resistance in these isolates were also
2. Materials and Methods
- Bacterial strains: Four representative MR P. aeruginosa isolates belonging to an endemic clone from the Hospital del Mar at Barcelona (Spain) were selected.
|
3. Results
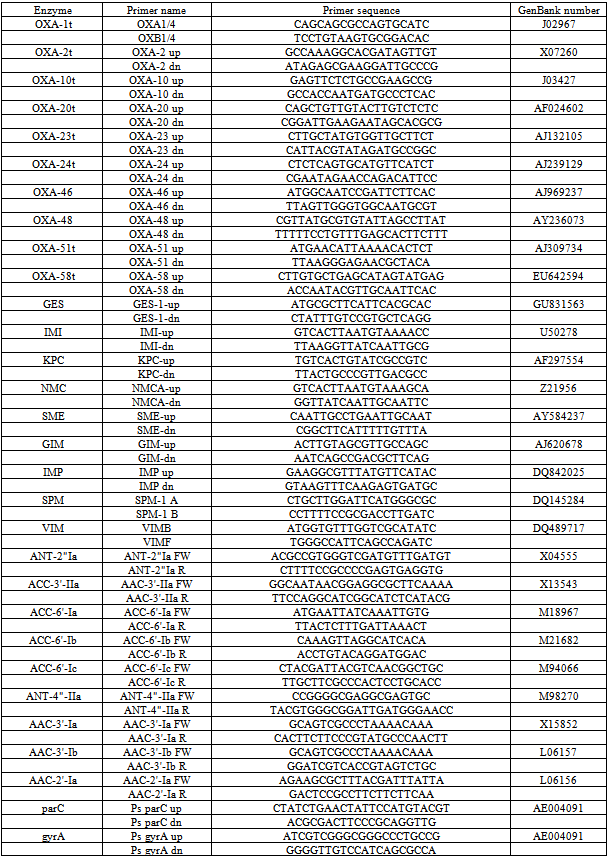
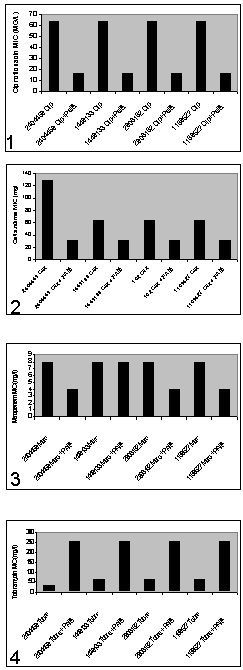
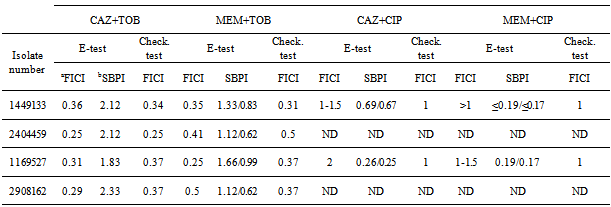
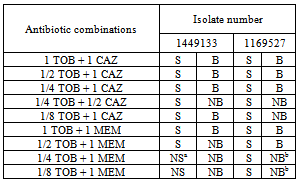
- In order to determine phenotypical susceptibility of the isolates studied, MicroScan® determination of MIC values (mg/L) gave the following results: Aztreonam 16; Ceftazidime 16 and >16; Cefepime 16; Piperacilline + tazobactam 32 and 64; Imipenem 8 and >8; CIP >2; Gentamicin >8; TOB >8; Amikacine 8 and 16; Colistine ≤2 mg/L.
4. Discussion
- In our hospital a prolonged endemism of MR P. aeruginosa has been observed. Colistine resistant or intermediate isolates were encountered, albeit in low number; the treatment of infections caused by such isolates is difficult and nowadays restricted to colistine and amikacine.On the other hand we tried to validate synergy testing by E-test as a rapid and easier tool to demonstrate synergy compared with the CB. Studied isolates showed resistance against all available antipseudomonal antibiotics except amikacin and colistine. Antibiotics for synergy testing were selected after reviewing the literature on the potentially active and less toxic antibiotic combinations for MR P. aeruginosa.The resistance pattern and the lack of carbapenemases and extended spectrum betalactamases suggested that resistance was mainly due to a derepresion of AmpC enhanced by OXA type betalactamases , up-regulation efflux system showed by the pump inhibitor effect of PAßN, reduction of OprD expression and alteration of topoisomerases and two aminoglycoside-modifying enzymes encoding resistance to kanamycin, gentamycin and tobramycin.
|
ACKNOWLEDGEMENTS
- This study was partially supported by the Ministry of Health and Consumer Affairs, Instituto de Salud Carlos III-Feder, Spanish Network for the Research Infectious Diseases (REIPI/RD06/0008/0013) and IPT-2011- 1402-900000 from Spanish “Ministerio de Ciencia e Innovación”
References
| [1] | Lister, P., Wolter, D., and Hanson, N. Antibacterial-Resistant Pseudomonas aeruginosa: Clinical impact and complex regulation of chromosomal encoded resistance mechanisms. Clin.Microbiol.Rev.22: 582-610. 2009. |
| [2] | Matthaiou, D.K., Michalopoulos, A., Rafailidis, P.I., Karageorgopoulos, D.E., Papaioannou, V., Ntani, G., Samonis, G. and Falagas, M.E. Risk factors associated with the isolation of colistin-resistant gram-negative bacteria: a matched case-control study. Crit. Care. Med. 36: 807-811. 2008. |
| [3] | Tam, V.H., Chang, K.T., Abdelraouf, K., Brioso, C.G., Ameka, M., McCaskey, L.A., Weston, J.S., Caeiro, J.P. and Garey, K.W. Prevalence, resistance mechanisms and susceptibility of multidrug-resistant bloodstream isolates of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 54: 1160-1164. 2010. |
| [4] | Giske, C.G., Monnet, D.L., Cars, O. and Carmeli, Y. Clinical and economic impact of common multidrug-resistant gram-negative bacilli. Antimicrob. Agents Chemother. 52: 813-821. 2008. |
| [5] | Montero, M., Sala, M., Riu, M., Belvis, F., Salvado, M., Grau, S., Horcajada, J.P., Alvarez-Lerma, F., Terradas, R., Orozco-Levi, M., Castells, X. and Knobel, H. Risk factors for multidrug-resistant Pseudomonas aeruginosa acquisition. Impact of antibiotic use in a Double case-control study. Eur. J. Clin. Microbiol. Infect. Dis. 29: 335-339. 2010. |
| [6] | Peña, C., Suarez, C., Tubau, F., Domínguez, A., Sora, M., Pujol, M., Gudiol, F. and Ariza, J. Carbapenem-resistant Pseudomonas aeruginosa: factors influencing multidrug-resistant acquisition in non-critically ill patients. Eur. J. Clin. Microbiol. Infect. Dis. 28: 519-522. 2009 . |
| [7] | Paterson, D.L. Impact of antibiotic resistance in gram-negative bacilli on empirical and definitive antibiotic therapy. Clin. Infect. Dis. 47: S14-20. 2008. |
| [8] | Aragón, L.M., Mirelis, B., Miró, E., Mata, C., Gómez, L., Rivera, A., Coll, P. and Navarro, F. Increase in β-lactam-resistant Proteus mirabilis strains due to CTX-M and CMY-type as well as new VEB- and inhibitor-resistant TEM-type β-lactamases. J. Antimicrob. Chemother. 61: 1029-1032. 2008. |
| [9] | Puig, M., Fusté, M.C. and Viñas, M. Outer membrane proteins from Serratia marcescens. Can. J. Microbiol. 39:108-111. 1993. |
| [10] | Beyer, R., Pestova, E., Millichap, J.J., Stosor, V., Noskin, G.A. and Peterson, L.R. A convenient assay for estimating the possible involvement of efflux of fluoroquinolones by Streptococcus pneumoniae and Staphylococcus aureus: evidence for diminished moxifloxacin, sparfloxacin, and trovafloxacin efflux. Antimicrob. Agents Chemother. 44:798-801. 2000. |
| [11] | Knapp, C. and Moody, J. A. Tests to assess bactericidal activity. In: Isenberg HD, ed. Clinical microbiology procedures handbook. Washington: American Society for Microbiology, 1992. |
| [12] | Balke, B., Hogardt, M., Schmoldt, S., Hoy, L., Weissbrodt, H. and Häussler, S. Evaluation of the E-test for the assessment of synergy of antibiotic combinations against multiresistant Pseudomonas aeruginosa isolates from cystic fibrosis patients. Eur. J. Clin. Microbiol. Infect. Dis. 25:25-30. 2006. |
| [13] | Sopirala, M.M., Mangino, J.E., Gebreyes, W.A., Biller, B., Bannerman, T., Balada-Llasat, J.M. and Pancholi, P. Synergy testing by Etest, microdilution checkerboard, and time-kill methos for pan-drug-resistant Acinetobacter baumannii. Antimicrob. Agents Chemother. 54: 4678-4683. 2010. |
| [14] | Milne, K.E.N. and Gould, I.M. Combination testing of multidrug-resistant cystic fibrosis isolates of Pseudomonas aeruginosa: use of a new parameter, the susceptible breakpoint index. J. Antimicrob. Chemother. 65: 82-90. 2010. |
| [15] | Shigeharu, O., Uematsu, T., Sawa, A., Mizuno, H., Tomita, M., Ishida, S., Okano, Y. and Kamiya, A. In vitro effects of combinations of antipseudomonal agents against seven strains of multidrug-resistant Pseudomonas aeruginosa. J. Antimicrob. Chemother. 52: 911-914. 2003. |
| [16] | Song, W., Woo, H.J., Kim, J.S. and Lee, K.M. In vitro activity of β-lactams in combination with other antimicrobial agents against resistant strains of Pseudomonas aeruginosa. Int. J. Antimicrob. Agents 21: 8-12. 2003. |
| [17] | Miller, M.H., Feinstein, S.A. and Chow, R.T. Early effects of β-lactams on aminoglycoside uptake, bactericidal rates, and turbimetrically measured growth inhibition in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 31:108-110. 1987. |
| [18] | Fish, D.N., Choi, M.K. and Jung, R. Synergic activity of cephalosporins plus fluoroquinolones against Pseudomonas aeruginosa with resistance to one or both drugs. J. Antimicrob. Chemother. 50: 1045-1049. 2002. |
| [19] | Thalhammer, F. and Hörl, W.H. Pharmacokinetics of meropenem in patients with renal failure and patients receiving renal replacement therapy. Clin. Pharmacokinet. 39:271-279. 2000. |
| [20] | Roberts, J.A., Kirkpatrick, C.M.J., Roberts, M.S., Robertson, T.A., Dalley, A. and Lipman, J. Meropenem dosing in critically ill patients with sepsis and without renal dysfunction: intermittent bolus versus continuous administration? Monte Carlo dosing simulations and subcutaneous tissue distribution. J. Antimicrob. Chemother. 64:142-50. 2009. |
| [21] | Kasiakou, S.K., Lawrence, K.R., Coulis, N. and Falagas, M.E. Continous versus intermittent intravenous administration of antibacterials with time-dependent action. Drugs 65: 2499-2511. 2005. |
| [22] | Craig, W.A. and Ebert, S.C. Killing and regrowth of bacteria in vitro: a review. Scan J. Infect. Dis. suppl 74:63-70. 1991. |
| [23] | Blaser, J., Stone, B., Groner, M., and Zinner, S.H. Comparative study with enoxacin and netilmicin in a pharmacodynamic model to determine importance of ratio of antibiotic peak concentration to MIC for bactericidal activity and emergence of resistance. Antimicrob. Agents Chemother. 31: 1054-60. 1987. |
| [24] | Lam, W., Tjon, J., Seto, W., Dekker, A., Wong, C., Atenafu, E., Bitnun, A., Waters, V., Yau, Y., Solomon, M. and Ratjen, F. Pharmacokinetic modelling of a once-daily dosing regimen for intravenous tobramycin in pediatric cystic fibrosis patients. J. Antimicrob. Chemother. 59: 1135-1140. 2007. |
| [25] | Prescott, W.A. Jr. and Nagel J.l. Extended-interval once-daily dosing of aminoglycosides in adult and pediatric patients with cystic fibrosis. Pharmacotherapy 30: 95-108. 2010. |
| [26] | Yuan, Z., Ledesma, K.R., Singh, R., Hou, J., Prince, R.A. and Tam, V.H. Quantitative assessment of combination antimicrobial therapy against multidrug-resistant bacteria in a murine pneumonia model. J. Infect. Dis. 201: 889-97. 2010. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML