-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Microbiology Research
p-ISSN: 2166-5885 e-ISSN: 2166-5931
2013; 3(2): 92-98
doi:10.5923/j.microbiology.20130302.06
Prevalence, Levels, and Relatedness of Listeria monocytogenes Isolated from Raw and Ready-to-Eat Foods at Retail Markets in Culiacan, Sinaloa, Mexico
Gloria Marisol Castañeda-Ruelas1, Nohelia Castro-del Campo1, Josefina León Félix1, José Benigno Valdez Torres1, Roberto Guzmán-Uriarte1, John B. Luchansky2, Anna C. S. Porto-Fett2, Bradley A. Shoyer2, Cristóbal Chaidez1
1Centro de Investigación en Alimentación y Desarrollo, A. C. Coordinación Culiacán. Carretera a Eldorado km 5.5, 80129 Culiacán, Sinaloa, México
2USDA/ARS/ERRC, 600 East Mermaid Lane, Wyndmoor, Pennsylvania, 19038, USA
Correspondence to: Cristóbal Chaidez, Centro de Investigación en Alimentación y Desarrollo, A. C. Coordinación Culiacán. Carretera a Eldorado km 5.5, 80129 Culiacán, Sinaloa, México.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Prevalence, levels, and relatedness of Listeria monocytogenes strains isolated from select raw and ready-to-eat foods at retail markets in Culiacan, Sinaloa, Mexico were determined during 2011. L. monocytogenes was isolated from 26 (14.4%) of 180 food samples. Raw chicken breast showed the highest occurrence (23.3%; 14/60), followed by ready-to-eat turkey frankfurters (11.7%; 7/60) and raw ground beef (8.3%; 5/60). Pathogen levels ranged from 0.23 to 110 MPNg-1. One isolate from each of the 26 positive samples was serotyped as follows: 1/2a (9 isolates), 1/2b (4 isolates), 1/2c (2 isolates), 3b (1 isolate), and 4b (10 isolates). Twenty-three of the 26 strains were resistant to a least one of 18 antimicrobials tested and were delineated into 11 antibiotic profiles. The 26 isolates were further delineated into 14 AscI pulsotypes. This data confirms a relatively high prevalence and levels of L. monocytogenes, most likely originated from various sources due to the low relatedness among isolates recovered from positive samples. Further studies are needed to identify harborage points and to develop interventions to reduce the prevalence of L. monocytogenes and decrease the risk of listeriosis from food purchase at retail markets in Culiacan.
Keywords: Listeria Monocytogenes, PFGE, Serotype, Antimicrobial Profile, Prevalence
Cite this paper: Gloria Marisol Castañeda-Ruelas, Nohelia Castro-del Campo, Josefina León Félix, José Benigno Valdez Torres, Roberto Guzmán-Uriarte, John B. Luchansky, Anna C. S. Porto-Fett, Bradley A. Shoyer, Cristóbal Chaidez, Prevalence, Levels, and Relatedness of Listeria monocytogenes Isolated from Raw and Ready-to-Eat Foods at Retail Markets in Culiacan, Sinaloa, Mexico, Journal of Microbiology Research, Vol. 3 No. 2, 2013, pp. 92-98. doi: 10.5923/j.microbiology.20130302.06.
Article Outline
1. Introduction
- Listeria monocytogenes (LM) is an important foodborne pathogen due to its widespread occurrence and the severity of listeriosis[1]. In the United States (U.S.) there are an estimated 1600 foodborne illnesses each year attributed to LM, with a high percentage of hospitalizations (>87%) and an overall mortality of 16%[2]. In Mexico, in 2010 the National Epidemiology Department reported 805 meningitis cases and 4,923,459 undefined gastroenteritis cases[3]; however, LM was not attributed to these illnesses, presumably because it was not tested for. In contrast, from 1982 to 1992, eight listeriosis cases were reported in Mexico, and 50% of these patients died: the infection source was not identified[4]. Ready-to-eat (RTE), meat, poultry, vegetables, and dairy products are vehicles for listeriosis transmission. In fact, the annual cost of food recalls related to LM is estimated around to $2.4 billion dollars in the U.S.[1]. In Mexico food recalls are not reported due to lack of regulatory oversight. From a regulatory perspective, the European Commission (EC Regulation No. 2073/2005) established a limit of 100 CFUg-1 of LM in foods with a refrigerated shelf life ≤5 days or with intrinsic factors justifying the absence of its growth. In contrast, for food where the pathogen is likely to be present under normal storage conditions or products that are stored for >5 days, zero tolerance (absent in 25g) is enforced[5]. The U.S. has a “zero tolerance” (≤1 CFU/g) policy for LM in RTE foods[6]. In Mexico, the official norms NOM-121-SSA1-1994[7]/NOM-091-SSA1-1994[8] establishes that LM must be absent in 25 g or ml of all cheese types and pasteurized milk, whereas other RTE foods are exempt from this requirement. The occurrence of LM has been reported worldwide in raw and RTE foods. For example, in Italy, Pesavento[9] reported a prevalence of 23.6% (148/1268) in raw food and 25% (7/28) in RTE foods. In Spain, Vitas[10] estimated the prevalence of LM at 8% (220/2745) in raw foods and 9.3% (87/940) in RTE foods. In the U.S., Gombas[11] reported that 1.8% (577/31,705) of RTE foods tested positive for the pathogen. In Mexico, listeriosis has not been associated with consumption of food, even though the bacterium has been isolated from food throughout Mexico. The fact the listeriosis is not a reportable disease is the primary factor for the lack data on foodborne listeriosis in Mexico. For example, LM was detected in 15% (18/120) of raw beef samples purchased from butcher´s shops in Nuevo Leon[12]. Silva[13] recovered LM from 7.9% (20/254) of frankfurters and ham products exhibited for sale in Baja California. Lastly, Saltijeral[14] and Moreno-Enriquez[15] estimated the prevalence of LM in Queso Fresco (QF) and retail markets in Mexico city and Sonora state at 15% (18/120) and 3.4% (5/149), respectively. Since food is a vehicle for transmission, surveillance for LM might prevent foodborne listeriosis or ensure for a more rapid and effective treatment. Likewise, detection of changes in antimicrobial resistance patterns may help to improve background data on antimicrobial resistance of strains and, in turn, treatment modalities for patients infected with LM[16]. Several subtyping methods have established relatedness among isolates and have been used to track outbreaks and identify niches of the bacterium. Currently, the ‘gold standard’ for subtyping LM is pulsed field gel electrophoresis (PFGE). The application of PFGE has been quite useful for outbreak prevention and identification via PulseNet[17].In 2007, in Culiacan, Sinaloa, Mexico the occurrence of LM in QF purchased at groceries was estimated at 9% (7/75)[18]; however, the occurrence of LM in other foods at retail markets has not been established. Culiacan city has about 858,638 inhabitants[19], a significant number of whom purchase foods from the three major retail markets within the city. Therefore, this study was undertaken to determine the prevalence, levels, and types of LM in foodstuffs from the three main retail markets in Culiacan city to establish baseline data on LM subtypes distribution that would ultimately have a positive impact on public health.
2. Materials and Methods
2.1. Sample Collection
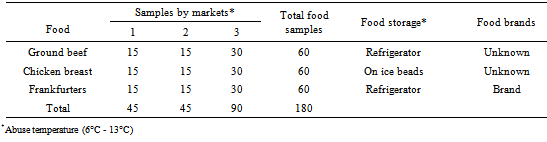
- A total of 180 food samples (Table 1) were randomly purchased from three municipal markets located in Culiacan, Sinaloa, Mexico, from January to August of 2011. The samples included raw meat (ground beef and chicken breast) and RTE turkey frankfurters packed under vacuum. The samples were transported in an icebox to the laboratory for analysis within 2 h after purchase. The owners individually operate the markets, food brands are commercialized, temperature is not monitored/controlled, food is purchased/sold by pieces, raw food is unpacked, and non-hygienic measures either from food handler personnel and facilities are observed.
2.2. Detection of LM
- Food samples were analyzed for prevalence of LM according to the USDA-FSIS method[20]. Presumptive colonies were further confirmed via LM-specific PCR by amplifying a 240-bp fragment of the hlyA gene[21]. One colony from each positive sample was retained for further characterization.
2.3. MPN Enumeration
- The LM positive samples were subsequently analyzed to determined pathogen levels using the Most Probable Number (MPN) technique and an accepted reference table according to the USDA-FSIS method[22]. A series of 3-tube and 4-dilution (up to 10-3) MPN series was performed. The MPN dilutions that screened positive (changed color to black) were streaked to on Oxford agar plates (Oxoid, Hampshire, England). Five LM-like colonies were confirmed by PCR[21].
2.4. Serotyping
- Serotyping was performed using a commercial somatic (O) and polyclonal flagellar (H) antisera (Denka Seiken Co. Ltd., Japan) according to the manufacturer’s instructions.
2.5. Antimicrobial Susceptibility Testing
- The LM isolates were tested for susceptibility to 18 antimicrobial agents used in veterinary and human therapy by a standard disk diffusion method of Kirby-Bauer[23]. Isolates were classified as resistant or susceptible to the antimicrobial according to the size of the inhibition halo as described in NCCLS[23]. The selected antimicrobials were apramycin (10 µg), amikacin (30 µg), streptomycin (25 µg), gentamicin (10 µg), kanamycin (30 µg), ampicillin (10 µg), imipenem (10 µg), amoxicillin-clavulanic acid (30 µg), ciprofloxacin (5 µg), cefoperazone (30 µg), ceftriaxone (30 µg), cephalothin (30 µg), ceftazidime (30 µg), chloranphenicol (10 µg), colistin (25 µg), nalidixic acid (30 µg), trimethoprim-sulfamethoxazole (1.25/23.75 µg), and tetracycline (30 µg) (Oxoid). Escherichia coli ATCC 25922 and Staphylococcus aureus ATCC 25923 were used as antimicrobial susceptible controls.
2.6. Pulsed Field Gel Electrophoresis Typing
- Genomic DNA was prepared in agarose plugs as described by Graves[17]. The DNA was digested using AscI (BioLabs, Beverly, MA). L. monocytogenes MFS1435 (serotype 1/2a) and Salmonella Branderaup H9812 were used as controls. Pattern images were acquired using a Bio-Rad Gel Doc system with the Multi-Analyst software program (version 1.1, Bio-Rad, Hercules, CA) and compared using the Applied Maths BioNumerics software package (version 4.0, Saint-Martins-Latem, Belgium). Pattern clustering was performed using the software BioNumerics, specifically the unweighted-pair group method (UPGMA), arithmetic averages, and the Dice correlation coefficient with a tolerance of 1%.
2.7. Statistics
- Descriptive statistics were calculated and correlations among categorical variables (food and market) were examined by Х2 analysis (Minitab® Statistical Software, Pennsylvania, U.S.), and a P value <0.05 was considered significant.
3. Results and Discussion
3.1. Prevalence and Contamination levels of LM in Retail Food
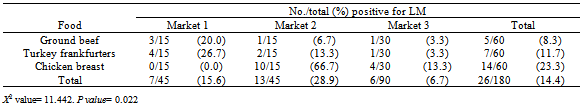
- Identification of harmful bacteria in foods can provide insight on the safety of retail foods and, therefore, the potential risk to consumer´s health. LM was isolated from 26 of 180 food samples, for an overall prevalence of 14.4% (Table 2). The correlation analysis confirmed that LM was associated to the type of food and associated market from which it was purchased (P= 0.022). For example, raw chicken breast showed the highest occurrence (23.3%) of LM, followed by turkey frankfurters (11.7%) and then ground beef (8.3%). Regarding the three markets, LM was isolated more frequently from market 2 (28.9%) compared to markets 1 and 3. However, it is important to highlight the nonhygienic measures of these markets, like the unpacked presentation of several foods, direct use of bare hands, personnel working with dirty utensils and surfaces, and the lack of refrigeration, all of which creates a higher risk for cross-contamination and growth of LM and others pathogens.The observed prevalence of LM reported herein is in general agreement with the reported incidence of LM in retail foods sold in Mexico[12, 13]. Previous studies focused on one type of RTE or raw food. However, the prevalence of LM in raw chicken was not previously documented in Mexico; a significant association of LM and raw chicken was found in the present study. Mena[24] reported rates of LM at 17% (3/17) in beef and 60% (9/15) in poultry raw samples in Portugal. These authors commented that raw meat represents an important reservoir for LM, this enhancing the risk for cross-contamination to occur at the retail level and at home. Although, there is a risk of listeriosis from raw food, especially related to cross-contamination by retailers and/or consumers, this risk would be reduced by the proper storage at retail and adequate handling and cooking by consumers. The persistence and relatively high occurrence of LM (11.7%) in turkey frankfurters (all positive samples were of the same commercial brand) indentified at the three markets in Culiacan suggests deficient control measures during production and retailing. Wallace[25] reported a prevalence of 1.6% (532/32,800) in frankfurters packages obtained from several commercial manufactures; however, a comparatively large number of packages of one brand of turkey frankfurters contained LM (16%; 473 of 2800 packages). Indeed, frankfurters are a higher risk product due to its association with LM and its extended shelf life, as well as the preference of some consumers to ingest frankfurters without reheating[26]. Turkey frankfurters, ground beef and chicken breast samples showed LM levels between 0.23-4.6 MPNg-1, 0.23-0.43 MPNg-1, and 0.23-110 MPNg-1, respectively. Generally, LM is detected at low levels (<0.3 MPNg-1) in both raw and RTE foods, but higher levels have been reported on occasion (>105 CFUg-1)[11, 27]. The recovery of LM in frankfurters is alarming, since frankfurters are RTE, and since amplification of the bacterium at home during extended shelf life (120 days), most likely due to an improper storage temperature[26]. Our data established a real consumer exposure to LM in retail foods, which could be higher as a result of the improper storage conditions at the markets (abuse temperature, unknown shelf life, open packaging). Ensuring consumer safety will require better training of producers and retailers about temperature control, good manufacturing practices, and sanitation programs to prevent the occurrence of LM and its potential growth to high levels in most food at retail[5].
3.2. Serotyping
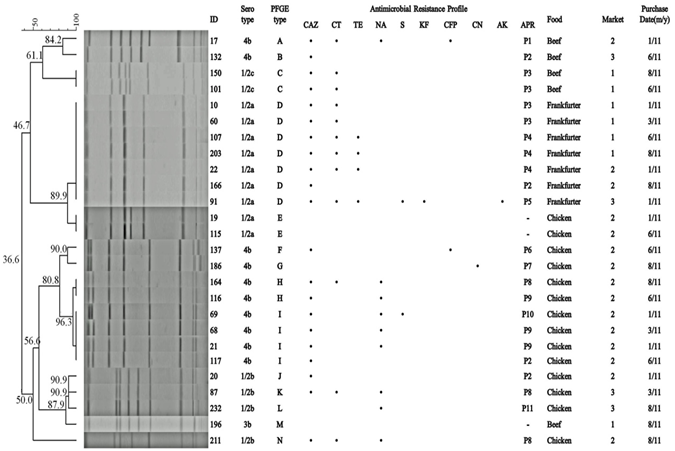
- A total of 26 LM strains (one isolate from each positive sample) were grouped into five different serotypes as follows: 4b (ten isolates), 1/2a (nine isolates), 1/2b (four isolates), 1/2c (two isolates), and 3b (one isolate). The distribution of serotypes among the foods and markets sampled are shown in Figure 1. In comparison, the total isolates belonged to serotype 1/2a were isolated from RTE turkey frankfurters, whereas the common isolates from raw meat belonged to serotype 4b. In general, our serological results are in agreement with previous study of LM isolated from retail raw and RTE foods[10, 28]. The majority of human listeriosis worldwide outbreaks have been linked to serotypes 4b, 1/2b, and 1/2a. Presently, serotypes 1/2a and 1/2c strains are classified as belonging to lineage II, which includes strains commonly found in foods and food environments, and are also associated with sporadic human clinical cases. In contrast, serotype 1/2b, 3b, and 4b strains belong to lineage I, which is more closely associated with human listeriosis outbreaks, especially serotype 4b, but, serotype 4b is not the serotype most frequently isolated from foods[1]. Also, these authors mentioned that the differences about the ability to be transmitted through foods and to cause human disease among lineages isolates are due to serotype 4b, they are more virulent than others serotype, while the linage II isolates seem to have better characteristics of adaptation to the environment and foods. In Mexico, there are not published reports on the serotypes isolated from clinical samples[4]. However, Saltijeral[14] reported serotypes 1/2a, 1/2b, and 4b isolated from Panela cheese sold in Mexico. Our findings identified the diversity and distribution of five serotypes among the foods and markets sampled, and the main occurrence of serotype 4b. The presence of the epidemiologically relevant serotype 4b indicates that the selected food may serve as infection sources to humans, particularly food that is not properly handled and/or stored.
3.3. Antimicrobial Susceptibility Testing
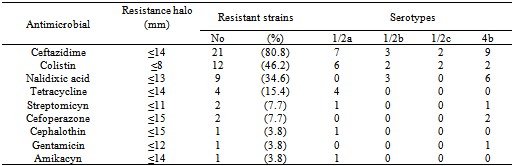
- Surveillance for antimicrobial resistant LM strains is useful to date the emergence of antimicrobial-resistant strains and to ensure effective treatment to human listeriosis. This study provides the first evidence of resistant LM strains isolated from raw and RTE foods retailing in Mexico; 88.5% (23/26) of LM strains showed resistance to at least one of 18 antimicrobials tested (Table 3), and were classified into 11 resistance profiles (Figure 1). Multidrug resistances were observed in 17 of 23 (73.9%) LM isolates. All isolates were susceptible to ampicillin, amoxycillin/clavulanic acid, apramycin, ceftriaxone, chloranphenicol, ciprofloxacin, kanamycin, imipenem, and trimethoprim-sulfamethoxazole. It should be noted that our strains displayed susceptible to the major classes of antimicrobials used locally in the listeriosis therapy. In Mexico, the standard therapy against listeriosis includes ampicillin (12-20 g/day) given intravenously, as well as ampicillin plus amikacin[4]. In Mexico, Kraus[4] isolated LM strains from eight immunosuppressed patients, and found that clinical LM strains were susceptible to ampicillin, amikacin, penicillin, and ceftriaxone. In spite of susceptibility of LM strains and the appropriate therapy provided to the patient, 50% of them died. Previously, Rodas-Suárez[29] reported isolation of LM strains from fish and estuarine water in Mexico that were resistant to gentamicin, dicloxacillin, cefuroxime, cephalothin, tetracycline, erythromycin, trimethoprim - sulfamethoxazole, penicillin, ampicillin, ceftazidime, and pefloxacin. Resistance to a diverse set of antimicrobials, especially antimicrobials used in listeriosis therapy has also been documented worldwide among LM strains isolated from raw meat and retail foods[9, 28]. It is known that antimicrobial used in humans and animals can appreciably influence the resistance of the bacterium. In this sense, Mexico´s laws allow the antimicrobials tested in this study to be used both as clinical drugs and as food ingredients for animals[30], but information on correct dose and type of use is scarce. Therefore, the continued surveillance on emerging resistances strains can prove useful to note changes in their resistance profile over time, and subsequently treat possible listeriosis cases in the study area.
3.4. Genetic Diversity Analysis by PFGE
- All LM isolates were fingerprinted using Ascl, with a cut-off level of 100% similarity (Figure 1). Of the 14 resulting PFGE patterns (A to N), nine were unique, being displayed by only one strain. The remaining five PFGE patterns were shared among two (C, E, H), four (I), and seven (D) strains. Our findings showed that PFGE patterns of LM strains recovered from food were persistent and diverse over time within the three markets (Figure 1). The PFGE results also suggest a potential association of an exclusive PFGE pattern with a specific food type. For example, pattern D obtained from turkey frankfurters was collected over the entire sampling period (January to August) and within the markets, indicating the persistence and widespread of this genotype in the region. We hypothesize that there was a single food producer supplying these markets. Alternatively, there could be a point source that contaminated each batch of frankfurters. Isolates from raw beef and chicken showed a distinct PFGE pattern (Figure 1), which may indicate different producers for these food categories or the acquisition of the genetic profiles as a result of a sporadic/point source of LM at the production or retail level. Previously, Chaidez[18] found a low genetic heterogeneity of LM strains isolated from QF in Sinaloa, and suggested that specific subtypes may be predominant in a food type as a consequence of common/point source of contamination. However, several authors reported the persistence and spread of LM strains displaying the same profile in different foods, brands, and places; even in a single processing plant, different genetic profiles were found as a result of deficiencies in the sanitary process which, in turn, allowed for colonization by the strains[31, 27, 28]. Moreno-Enriques[15] point out that final food products, environmental samples, and food contact surfaces, both in processing and retail level as sources of predominant and persistence LM types. In particular, local foods products represent a vehicle for certain clonal LM strains, therefore, the PFGE typing of LM recovered from foods at retail markets in Mexico can establish relatedness among strains and identify sources that harbor the pathogen which, in turn, can lessen the likelihood and/or severity of foodborne listeriosis.
|
|
|
4. Conclusions
- The occurrence of LM in foods sold at public retail markets at Culiacan city, and the distribution of certain PFGE clonal types belonging to major clinical serotypes (1/2a, 1/2b and 4b) poses a risk for consumers of these foods, since some strains could be responsible for foodborne listeriosis, especially in RTE food. The low microbiological quality of foods at markets in Culiacan, suggests that the bacterium is likely to be found in other markets in Mexico with similar sanitary conditions, justifying worker/vendor training and further research on the persistence of the pathogen, as well as the application of simple and cost effective corrective actions at market levels. Consequently, continuous surveillance of LM in retail markets around Mexico will help identify pathogen sources and promote the development of best practices for processing and selling food. It should also improve the legislative framework that governs the presence of the pathogen in food. Lastly, these data confirm that good hygienic practices and temperature control are critical for prevention and control of LM in food at retail markets.
ACKNOWLEDGEMENTS
- We extend our gratitude to Célida Martínez Rodríguez from the Centro de Investigación en Alimentación y Desarrollo, A. C. for providing technical assistance.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML