-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Microbiology Research
p-ISSN: 2166-5885 e-ISSN: 2166-5931
2013; 3(2): 87-91
doi:10.5923/j.microbiology.20130302.05
Chemical Composition and Antimicrobial Effect of the Essential Oil of Pelargonium graveolens (Geranium Rosat) Grown in Butare (Rwanda) Towards Formulation of Plant-based Antibiotics
Kabera Justin1, Mugiraneza Jean Pierre1, Chalchat Jean Claude2, Ugirinshuti Viateur1
1Phytomedicines and Life Sciences Research Programme, Institute of Scientific and Technological Research (I.R.S.T.), P.O Box 227 Butare, Rwanda
2University of Clermont-Ferrand, P.O. Box 185, 63006 Clermont-Ferrand cedex, France
Correspondence to: Ugirinshuti Viateur, Phytomedicines and Life Sciences Research Programme, Institute of Scientific and Technological Research (I.R.S.T.), P.O Box 227 Butare, Rwanda.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
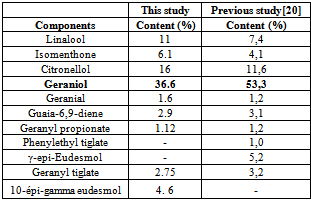
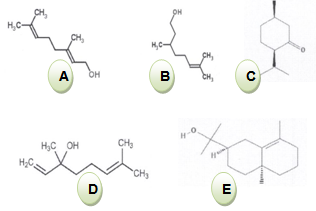
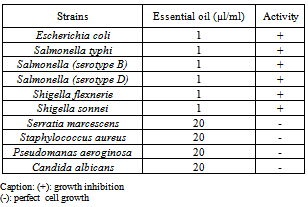
The essential oil obtained by hydrodistillation of the leaves of Pelargonium graveolens was analysed by GC/FID and GC/MS methods. Individual components of the oil were identified by their mass spectra which were compared with previously published works. About sixty one compounds were identified, among which the major components were geraniol (36. 6 %), citronellol (15.9%), linalool (11.0%), isomenthone (6.1%) and 10-épi-gamma eudesmol (4.6%). The essential oils of P. graveolens revealed to exhibit a significant antibacterial activity against Escherichia coli, Salmonella typhi, Salmonella serotypes B and D, Shigella flexnerie and Shigella sonnei even at small concentrations (1µl/ml). Quite the reverse, this investigation showed that the essential oil of P. graveolens has no effect on the fungus Candida albicans, and the bacteria such us Staphylococcus aureus, Serratia marcescens and Pseudomanas aeroginosa. They remain resistant at even high concentrations of its essential oil (20 µl/ ml). The results obtained in the present study indicate the possibility of exploiting the essential oil of P. graveolens to fight against so many infectious human diseases in Rwanda. At this level of knowledge, the formulation of synthetic drugs against some human infectious diseases is believable. However, further investigations are required to make the medical exploitation of this plant successful
Keywords: Antimicrobial Activity,Butare,Chemical Composition, Formulation of Antibiotics, Pelargonium Graveolens, Rwanda
Cite this paper: Kabera Justin, Mugiraneza Jean Pierre, Chalchat Jean Claude, Ugirinshuti Viateur, Chemical Composition and Antimicrobial Effect of the Essential Oil of Pelargonium graveolens (Geranium Rosat) Grown in Butare (Rwanda) Towards Formulation of Plant-based Antibiotics, Journal of Microbiology Research, Vol. 3 No. 2, 2013, pp. 87-91. doi: 10.5923/j.microbiology.20130302.05.
Article Outline
1. Introduction
- Originating in South Africa according to[26] Pelargonium graveolens is a plant of the family of Geraniaceae introduced in Europe at the turn of the fourteenth century and in the Reunions Island in about 1870 where its farming took a great importance and this made it mainly the capital of geranium (P. graveolens). In Rwanda, during the colonial time, the geranium was cultivated in Byumba prefecture especially in Nyagafunzo, Mukarange, Kiyombe, Cyungo and Kibari districts, in Gisenyi prefecture especially in Pfunda, in Cyagugu and in Rural Kigali prefecture especially in Ndera, Kabare regions[20]. The essential oil of geranium is much known for its miraculous repulsive properties on mature mosquitoes from where its interest in the fight against paludism has been taken[29]. In addition to these properties, it exhibits also astringent, disinfectant, bactericidal, healing, antalgic, haemostatic, tonic, parasiticidal and anti-diarrhoeac properties[11]. In this study, the experiments consisted in testing the activity of the oil extracted from the dried leaves of P. graveolens grown in Mukoni (Butare, Rwanda) on various microorganisms especially bacteria and fungi. E scherichia coli was one of microorganisms subjected to this study and one of the members of human intestinal microbial flora. It is not necessarily pathogenic but it can be at the base of urinary infections via the production of the enterotoxins[3]. Salmonella spp found in the intestines of several animals are potentially pathogenic. Salmonella typhi is responsible for the typhoid fever and other Salmonella spp such as S. choleraesuis, and S. enterocolitis and S. enteritidis cause for salmonellosis[16]. Some bacterial strains of genus Shigella are found in human intestines and are the responsible for the bacillary dysentery or shigellosis[28]. Candidas albicans, one fungal strain subjected to this investigation, is one of fungal strains found in human body, especially on the mouth and genital mucous membrane. It is not dangerous but it causes sometimes the candidiasis (candidosis) in human beings [7],[23].During several centuries, the synthetic chemicals were used to control various plants and animal diseases. However, the resistance of the pathogens to these chemicals and the accumulation of the undesirable residues in the environment and their secondary effects, when they are used as pesticides or drugs, raised many discussions[21],[8]. This last is known as the phenomenon of bio-accumulation [9]. Even with low dose, the presence of these toxic substances in the environment represents a danger to the living organisms through the food chains. These last years, this challenge disputed the use of synthesized chemicals and led the scientists to direct their research towards other alternatives. From then on, the use of bioproducts including the essential oils has been promoted. They are abundant in nature and their use respects other species of organisms in the ecosystem and among them, the essential oils are being tested in Rwanda by the Institute of Scientific and Technological Research (IRST).The essential oils are mixtures of more or less many components that, alone or in synergy, impart to most of essential oils the biocidal properties [9],[8],[12]. Thus, having the same constraints in Rwanda, we carried out a research project leading to the use of essential oils of Pelargonium graveolens as bio-products to provide the people concerned by the health with the alternative way to reduce the human infections caused by microbes from the nature.
2. Materials and Methods
2.1. Plant Material
- After the dew was removed by the morning sun, the plant material that consisted of the fresh leaves of Pelargonium graveolens were collected from the gardens of medicinal plants established by the Pytomedicines and Life Sciences Research Program of IRST in Mukoni at Butare, the Southern province of Rwanda. The collected leaves without dew were stored for further oil extraction.
2.2. Microbial Material
- The microbial strains were as Escherichia coli, Salmonella typhi, Salmonella B, Salmonella D, Shigella flexnerie, Shigella sonnei, Serratia marcescens, Staphylococcus aureus, Pseudomanas aeroginosa and Candida albicans. They were collected from the laboratory of the University Teaching Hospital of Butare (CHUB), multiplied and used in this investigation. In this hospital, these bacterial strains are usually isolated from the faeces and other samples of the patients and stored at the laboratory for various analyses.
2.3. Extraction of Essential Oil
- The extraction of essential oils was performed in Clevenger apparatus for 2 hours on 100 g of air-dried leaves for many rounds, in the chemistry laboratory of the Institute of Scientific and technological Research (IRST). The leaves were washed with fresh water and introduced in clean flask. The water was added so as to cover the mixture and the grains of pumice were introduced in order to regulate the boiling and homogenize temperature inside the flask. The flask was connected to the distillation system and brought to boil. The organic phase was recovered by decantation method from hydro-distillate. This organic phase was the solution containing the essential oil we looked for. I was stored in hermetically sealed dark glass flask with rubber lids to protect them from light and kept under refrigerator at 4℃ until use [10],[14].
2.4. Chemical Analysis of the Essential Oil
- The analyses were carried out on GC of mark Hewlett Packard HP 6890 equipped with an injector split/splitless (280C), with a capillary tube HP-5 capillary column (30m X 0.25 mm, film thickness, 0.25 µm). The increase of the temperature was 50℃ (during 5 min) to reach the maximum of 300℃ at the rhythm of 5℃/min. The helium was used as the carrier gas with speed of 1.0 ml/min. The injection of the sample consisted of 1µl of the oil diluted to 10% (v/v) with the acetone. The GC/MS analyses were performed on Hewlett Packard 5973/6890 systems that operates in EI mode (70 eV), capable equipped with a split/splitless injector (280℃), a split ratio 1:30, using two different columns: a fused silica HP-5 MS capillary column (30 m x 0.25 mm, cinematographic thickness 0.25 µm), and a HP-Innowax capillary column (60 m x 0.25 mm, cinematographic thickness 0.25 µm). The temperature program for the HP-5 MS column was 50℃ (5min) rising to 300℃ at the rate of 5℃/min and for the HP-Innowax column, 50°-250℃ at the rate of 5℃/min. Helium was used as the carrier gas at a flow rate of 1.0 ml/min[1]. The compounds were identified by their time of retention and the indices of Kovats compared with the data from the literature.
2.5. In Vitro Antibacterial and Antifungal Activity
- The microbial stocks were collected according to the methods described by. After the culture of a complex sample on SS-agar medium, a colony isolated from enteropathogens culture was also transferred on new SS-agar medium radially with a platinum loop carrying the cotton. A volume of essential oil was incorporated in pre-prepared medium until 10 ml as final concentration. After the agitation with a vortex, the hot mixture was poured in sterile Petri dishes. The Enterococcus were cultured on SS-agar medium, Candida albicans was put on the Sabouraud agar medium while Serratia marcescens, S. aureus, P. aeroginosa were grown on Muller-Hinton medium[25],[2]. After the isolation and identification, the inoculum was mixed with the liquid medium and the mixture was incubated at 37℃ during 24 hours. The culture was performed by striation method by and the hermetically closed dishes were then kept in a drying oven at 37℃ during 24 hours, except C. albicans which was kept during five day.
2.6. Biological Safety
- The manipulation of these pathogenic germs required high biological safety level of the laboratory in which this work was performed. It concerns the biosafety measures, safety manual, management of accidents and incidents, management of staff health, monitoring of safety systems and maintenance of various safety records[30]. Although the safety in facility design was taken into account on initial planning and set-up of the laboratory, the ongoing assessments were carried out to ensure suitability for the work undertaken. The elements addressed include the location and layout of laboratory, air flow and ventilation, material of work surfaces with respect to type of work and disinfection considerations and sanitation and handwashing facilities. The safety assessment included also protective clothing, gloves, goggles, etc, and examples of containment equipment include biological safety cabinets and centrifuges with sealed buckets. These equipments were removed when contaminated or when their use was no longer required, with proper decontamination before re-use or disposal. Duting this work, the manipulators adopted good microbiological techniques, maintained personal hygiene, stored the specimens and isolates of microorganisms with corresponding level of access control and maintenance of inventory and decontaminated properly and disposed as appropriate the infectious materials and waste. The contingency plans and procedures for accidents and incidents, for example, spillage of pathogens, failure of biological safety cabinet and autoclave, were in place. To ensure the good health of staff, the pre-employment check and the provision of immunization where indicated[29].
3. Results and discussion
|
|
4. Conclusions
- The essential oil of geranium (Pelargonium graveolens) is rich in geraniol, which makes it a part of the family of the phenols which are known for their interesting antibacterial property. In this study, this essential oil expressed a significant antibacterial activity against gram-negative bacteria. Contrarily, the gram-positive and few gram- negative bacteria and the fungus Candida albicans expressed a significant resistance to this essential oil. This study contributed a lot to the establishment of eco-friendly new drugs. In fact, besides the repulsive properties for mosquitoes of the essential oil of geranium, the results of this study showed that this plant offers the possibilities to formulate a new antibacterial product against many bacteria responsible for various human infections
ACKNOWLEDGEMENTS
- We are greatly indebted to the Institute of Scientific and Technological Research (IRST) of Rwanda for financial as well as material support. The authors thank the University of Clermont-Ferrand in France for their priceless contribution and experience sharing during this study and the authorities of Butare University Teaching Hospital (CHUB) for their supply of microbial strains.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML