-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Microbiology Research
p-ISSN: 2166-5885 e-ISSN: 2166-5931
2013; 3(2): 83-86
doi:10.5923/j.microbiology.20130302.04
Effect of Drought on Microbial Growth in Plant Rhizospheres
Sheela Reuben, Elvagris Segovia Estrada, Han Ping
Singapore Delft Water Alliance, Faculty of Engineering, National University of Singapore, 117577, Singapore
Correspondence to: Sheela Reuben, Singapore Delft Water Alliance, Faculty of Engineering, National University of Singapore, 117577, Singapore.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
The functional performance of rhizospheral bacteria is often subjected to adverse environmental pressures such as drought. In this study, differential bacterial populations were observed in rhizospheres of plants and the size and numbers of these bacteria were greatly influenced by water deprivation. Although drought caused substantial reduction in rhizosphere microorganisms, the restoration of favorable conditions brought about good recovery and the extent of this recovery was greatly influenced by the plant species.
Keywords: Rhizosphere Microbes, Drought, Microbial Counts
Cite this paper: Sheela Reuben, Elvagris Segovia Estrada, Han Ping, Effect of Drought on Microbial Growth in Plant Rhizospheres, Journal of Microbiology Research, Vol. 3 No. 2, 2013, pp. 83-86. doi: 10.5923/j.microbiology.20130302.04.
Article Outline
1. Introduction
- In an effort to develop garden cities, many tropical countries have adapted to functional green systems for remediation and water retention which are referred to as “water sensitive urban designs” (WSUD). Bioretention systems are one such WSUD which are employed in bioretention swales and bioretention basins that may be located within parkland areas, car parks or along roadway corridors which is currently being implemented in Singapore. Bioretention systems operate by filtering stormwater runoff through densely planted surface vegetation and then percolating runoff vertically through a prescribed filter media [1]. During infiltration, fine particulates are trapped and dissolved pollutants are removed by adsorption to filter media or by absorption or uptake by plants and/or microbial community in the plant-soil environment. The plant-microbe relationships in these systems are critical for effective bioremediation and water retention. Abiotic factors such as drought tilt the balance of these ecosystems making them subfunctional. Rhizospheres are ecological niches which are integral to many biochemical reactions. These regions surrounding the roots are influenced by exudation. Microbes play a critical role such as plant growth promoters or acts as pathogens inhibiting plant growth and also take part in microbial degradation of natural or synthetic compounds[2]. Plant-growth-promoting rhizobacteria (PGPR) elicit systemic tolerance to abiotic stress such as drought[3].It is reported that microbial biomass in soil carbon increases in response to drought with plant species composition being responsible for more than 90% of the variation in enzyme activities involved in carbon and nitrogen cycles[4]. However, very little is known regarding the microbial abundance in different rhizospheres and the effect of drought on them.In this study, different plant rhizospheres were sampled to compare the differences in abundance of microorganisms. Comparison between drought affected plant rhizospheres and well watered rhizosphereswere performed to understand the survival of microbial populations after drought stress and revival on onset of favorable conditions.
2. Materials and Methods
2.1. Sample Collection and Processing
- Samples were collected from 21 different plants and unplanted soil (control) from bioretention tanks, in sterile 50 ml tubes and immediately frozen until processing. Soil was collected from three replicate plant rhizospheres from the bioretention tank and mixed well to make a composite sample. Direct counting of microorganisms was performed based on published protocol[5]. Briefly, 2 g of the soil was weighed and 5 ml of 10% methanol was added to it. Methanol helps to break up the exopolymeric substances, which are mainly composed of polysaccharides and entrap the surface-associated bacteria. It was then sonicated at 35C for 15 minutes using a water bath sonicator (Rocker Soner 210, Australia) followed by centrifugation at 2000rpm for 1 min (Sorvall Legend X1R, Thermo Scientific, Germany). Five hundred µl of the supernatant were filtered through a black 0.2 µm polycarbonate filter (Nuclepore, 25 mm diameter, shiny side up) and stained as described below. Mounting media was prepared using mowiol 4-88, (Polyscience, USA) and glycerol (for fluorescence microscopy, Merck)[5]. The staining solution SybrGreen 1 was diluted in a ratio of 1: 100. Nine microlitres of the SybrGreen I staining and mounting medium were placed in the middle of a cover slip (22 mm X 22 mm), which was then put upside down on the filter. Finally, the cover slip was pressed carefully onto the slide by tweezers to dispense the staining solution equally over the filter. The slides were incubated for 30 min before imaging on a confocal laser scanning microscope (LSM Meta 510, Carl Zeiss). All prepared slides were imaged on the same day to avoid fading of the stain due to storage. The number of microbes was determined in samples fromrhizospheres of well watered plants, plant subjected to drought (2 months) and recovery (1 month).
2.2. Data Analysis
- All statistical analysis was performed using Microsoft Excel. Microbial counts were calculated using 15 frames of 100 sqµm and calculated per disc. Counting was performed using Image Pro software (Media Cybernetics, USA).
3. Results
3.1. RhizosphereMicrobialDiversity and Plant Types
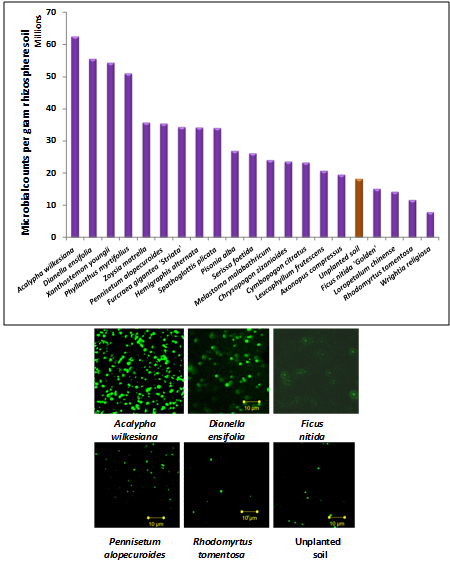
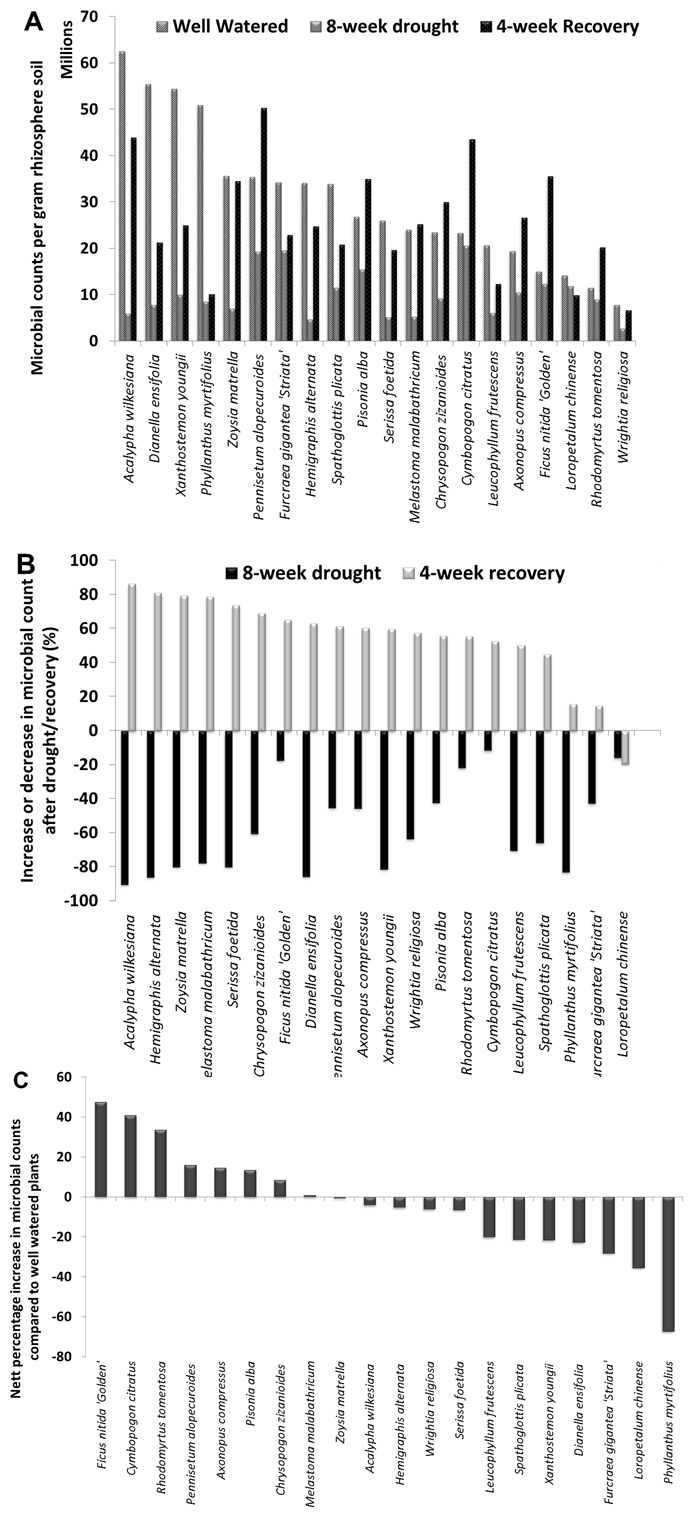
- The microbial abundance study of rhizosphere soils of 20 selected tropical garden plants was conducted. Diversity of microbial communities varied between the different plant types. Bacterial abundance in the rhizospheres varied according to the plant type (Figure 1A). A few representative images show the variation in number of microorganisms (Figure 1B). The number of microbes in the different rhizospheres ranged from 6.25x107 to 7.9x106cells/g soil. The unplanted soil showed counts equivalent to 1.8x107cells/g soil.
3.2. Effect of Drought on RhizosphereBacterial Counts
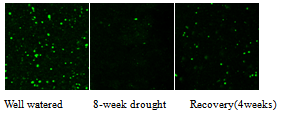 | Figure 2. Effect of drought on bacterial counts. Confocal images of bacterial counts in rhizospheres of H. alternatain well watered, drought and recovery conditions |
4. Discussion
- Bacteria utilize carbon sources exuded by roots for their growth. As expected, there was significant increase in the bacterial numbers in the rhizosphere region in comparison to bulk soil communities. This couldbe attributed to the availability of additional nutrients in the rhizosphere region due to root exudations. Bacterial communities are largely dependent on the type and amount of exudations from the plants. The difference in the type and amount of exudation lead to differences in the type of bacterial communities for the various plant types [6,7].When abiotic disturbances such as drought occur, there are further changes in the bacterial community composition and abundance [4]. This depends not only on the direct effect of physical stress on the microbes but also indirectly due to differences in carbon availability resulting from root exudation pattern changes that reflect plantstress. In this study, though root exudations have not been quantified, the effect of these changes on bacterial size and number has been studied. Bacteria decreased sharply when subjected to drought conditions and subsequently revived when favourable conditions were restored. This may be a consequence of inhibition and/or killing of sensitive species and selection of tolerant species by the disturbances applied [8]. Though all plants revived after drought stress only about 50% of the tested plants showed a positive increase in bacterial counts. Some plants surprisingly showed an increase in the bacterial counts. One of the possible explanations could be proliferation of fast-growing species after environmental soil conditions had been restored. These proliferating species may be specific to certain plants resulting in differential recovery of bacterial counts in rhizospheres.Rhizosphere bacteria are known to be involved in a variety of functions such as disease resistance [9]and plant growth enhancementand also help plants to survive drought stress [3,10]. Changes in the composition and abundance of bacterial communities may affect the biochemistry and functional roles in the rhizosphere ecosystem. Differential abundance and composition of microbes in rhizospheres could therefore also affect the bioremediation capacities of the water sensitive urban design (WSUD) systems. Since, bacterial counts for different horticultural plants were found to be significantly different from each other, bacterial abundance and functionalities in different plant rhizospheresshould be considered for plants when implementing phytoremediation strategies.
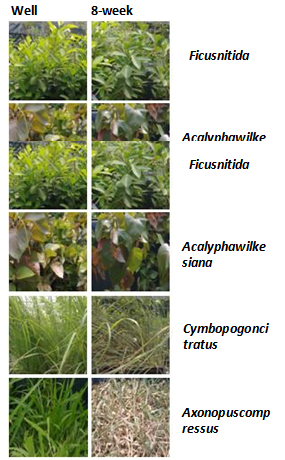 | Figure 5. Effect of drought on plants showing plants that were least affected to most affected by drought conditions |
5. Conclusions
- Differential bacterial populations were observed in plant rhizospheres which are affected by abiotic factors such as drought that greatly influence the size and numbers of bacteria in the rhizosphere. Recovery of rhizosphere bacteria is dependent not only on the return of favourable conditions but the regrowth of tolerant, proliferative species. In conclusion, the reduction and revival of certain bacteria is evident in the rhizosphere region. The differential microbial numbers may affect remediation capacity of bioretention systems and hence is an important factor to consider while growing different types of plants for water sensitive urban designs in tropical countries.
ACKNOWLEDGEMENTS
- The authors gratefully acknowledge the support and contributions of Singapore-Delft Water Alliance (SDWA). The research presented in this work was carried out as part of the SDWA’s Plant selection study for application in bioretention systems funded by Centre for Urban Greenery and Ecology (R303-001-017-490).
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML