-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Microbiology Research
p-ISSN: 2166-5885 e-ISSN: 2166-5931
2013; 3(2): 79-82
doi:10.5923/j.microbiology.20130302.03
Isolation, Identification and Susceptibility Patterns of Moraxella Catarrhalis among Children with Otitis Media in Khartoum State
Yousif Fadlalla Hamedelnil1, Zeinab Ahmed Ali Alharith1, Eltayib Hassan Ahmed-Abakur2
1Department of Microbiology, Faculty of Medical Laboratories Sciences, Sudan University of Sciences and Technology, Sudan
2Department of Microbiology, Faculty of Medical Laboratories Sciences, AlzaeimAlazhari University, Sudan
Correspondence to: Eltayib Hassan Ahmed-Abakur, Department of Microbiology, Faculty of Medical Laboratories Sciences, AlzaeimAlazhari University, Sudan.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
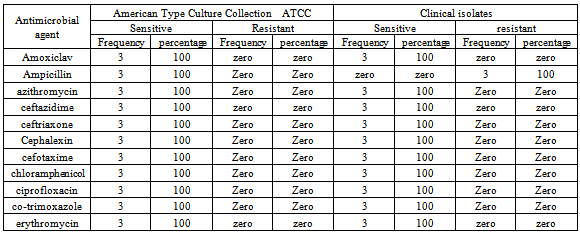
Otitis media is the most frequent infection associated with Moraxellacatarrhalis in children and comprises inflammation of the middle ear accompanied by a liquid effusion. The present study was aimed to determine the frequency and susceptibility patterns of M.catarrhalis in children with otitis media in Khartoum State, during the period from January to March 2011. A total of 110 specimens of middle ear discharge were collected by sterile swabs from Khartoum National Centre of Ear, Nose and Throat (ENT), Head and Neck Surgery.Specimens were cultured on chocolate agar, sheep blood agar and MacConkey agar. Ninety (82%) specimen showed significant growth while twenty (18%) did not show any growth. The identification of M.catarrhaliswas determined based on colonial morphology, Gram stain and a numberof biochemical tests. The susceptibility test was carried out using Kirby-Bauer disc diffusion method.Three (3.3%) isolates were positive for oxidase, catalase, DNase, tributyrin tests and reduced nitrate to nitrite and therefore, they were identified as M.catarrhalis. The clinical isolatesM.catarrhalis were found to be sensitive to amoxiclav, azithromycin, ceftazidime, ceftriaxone, cephalexin, cefotaxime, chloramphenicol, ciprofloxacin, cotrimoxazole and erythromycin and resistance to ampicillin which might be due to the production of β-lactamase enzyme.
Keywords: Moraxellacatarrhalis,Children, Susceptibility Patterns
Cite this paper: Yousif Fadlalla Hamedelnil, Zeinab Ahmed Ali Alharith, Eltayib Hassan Ahmed-Abakur, Isolation, Identification and Susceptibility Patterns of Moraxella Catarrhalis among Children with Otitis Media in Khartoum State, Journal of Microbiology Research, Vol. 3 No. 2, 2013, pp. 79-82. doi: 10.5923/j.microbiology.20130302.03.
Article Outline
1. Introduction
- Otitis media is inflammation of the middle ear, most commonly caused by the buildup of fluid behind the ear drum, as a result of a blockage to the Eustachian tube. It is more common in children, as their Eustachian tube is shorter and more horizontal than adults and is made up of more flaccid cartilage, which can impair its opening. Otitis media can cause a mild to moderate hearing loss, due to the fluid interfering with the transmission of sound through to the inner ear. It can often affect the tympanic membrane causing it to retract or become inflamed. The fluid can cause the tympanic membrane to bulge and become inflamed and occasionally the tympanic membrane will perforate[1].Otitis media is the most frequent infection associated with Moraxellacatarrhalis in children and comprises inflammation of the middle ear accompanied by a liquid effusion. Approximately 50% of children will have experienced at least one episode of acute otitis media (AOM) by their first birthday, this proportion rising to 70% by 3 years of age, representing a tremendous disease burden for this age group and necessitating the widespread use of antibiotics[2].Moraxella catarrhalisis a Gram-negativenon-encapsulated diplococcal bacterium belonging to the family Moraxellaceae. The genus Moraxella actually comprises both coccoid and rod-shape bacteria, and the classification of M. catarrhalishas been rather complex, the bacterium being alternatively named Branhamellacatarrhalis, Moraxella (Branhamella) catarrhalis, and now the preferred Moraxella catarrhalis[3, 4]. For the most of 20th century, M.catarrhalis was considered a saprophyte of the upper respiratory tract associated with no significant pathogenic consequences[5]. Currently it has been proven to be a pathogen in its own right with global isolates originating from two major increased potential to bind to human epithelial cells and an older lineage. Person -to- person spread through inhalation is not considered to be the mode of transmission, while hand- to hand nosocomial spread may be common in certain setting[6]. M. catarrhalishas been regarded as the third most important bacterial agent of acute otitis media in children, after S. pneumoniaeand H. influenzae[7, 8].Several reports showed an increased proportion of M. Catarrhalis isolation from the middle ear fluid (MEF) in acute otitis media (AOM).Kilpi et al.,[9], have reported an increase from 10% to 23% within 15years, and a similar pattern has also been reported in the United States. In Costa Rica, the prevalence of M. Catarrhalis isolated from the MEF of children with AOM aged 3–144 months increased from 2.5% of all pathogens during 1992–1997 to 7% during 1999–2004 and was most commonly found in children aged less than 24 months during the dry season[6].The financial impact on global health care systems of the high incidence of M. catarrhalis colonization and disease is significant, and consequently, several research groups are currently involved in identifying and assessing the usefulness of putative M. catarrhalis vaccine candidates[10]. However, Moraxellacatarrhalis is estimated to be responsible for 3-4 million cases of otitis media annually, with an associated health care cost (direct and indirect) of $2 billion each year[11].Bacterial resistance to antimicrobial agents has become an increasing problem in the treatment of otitis media. A multicenter surveillance study, carried out in Asia and Europe, demonstrated a high prevalence of antimicrobial resistance among respiratory pathogens and important differences in antimicrobial resistance profiles between countries. Pathogens that cause acute otitis media become resistant to commonly used antibiotics. The increasing rates of antibiotic resistance are due to repeated exposure of these bacteria to antibiotics and geographic spread of resistant strains. The rapid emergence of multidrug resistant otitis media in developing countries is a new potential threat to the survival of newborn babies and children[12].Unlikely our African countries lack the authentic data documentation system so it is very difficult to trace such information. According to the best of our knowledge this is first report of M.catarrhalis as pathogenic bacterium infected middle ear in the Sudan. The present study was a cross sectional study conducted to determine the frequency and susceptibility of M.catarrhalis in children (under 15 years) with otitis media during the period from January to March 2011 in Khartoum National Centre of ENT, Head and Neck Surgery.
2. Materials and Methods
2.1. Sample Collection and Isolation
- Samples were collected from 110 children who their parent agreed to participate in this study. Swabs samples were collected from the middle ear of infected children using sterile cotton wool swabs and transported in Amies Transport Media to the laboratory for diagnosis in the same day of collection. The specimens were inoculated under aseptic condition into MacConkey agar, sheep blood agar, chocolate agar and nutrient agar, and incubated at 370C for 48 hours at presence of 10% CO2.
2.2. Identification
- The identification of M.catarrhalis was based primary on colonial morphology, Gram stain and a number of biochemical tests namely; Oxidase, Catalase, Nitrate Reduction and DNase. Moraxellacatarrhalis was differentiated from Neisseria using DNase, tributyrin test and growth on nutrient agar at 35℃[13, 14, 15]. Several others biochemical tests were used to identify the causative agents of OTM other than M.catarrhalis (data not shown). Three control strains of M.catarrhalis ATCC2 5240, 25238 and 23246 from the American Type Culture Collection (ATCC) were used to confirm the results obtained by clinical isolates.
2.3. Antibiotic Susceptibility Test
- The susceptibility test was done by Kirby-Bauer disc diffusion commonly used method.The following eleven antimicrobial impregnated disks were used: amoxiclav, ampicillin, azithromycin, ceftazidime, ceftriaxone, cephalexin, cefotaxime, chloramphenicol, ciprofloxacin, co-trimoxazole and erythromycin.
3. Results
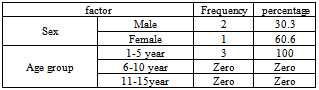
- Out of 110 specimens, 90 (82%) showed significant growth whereas 20 (18%) sample displayed no or insignificant growth. The colonial morphology was used as primary identification tool that differentiates M.catarrhalis from other microorganisms. M.catarrhalis was grown well on sheep blood agar and chocolate agar but not on MacConkey agar. On sheep blood agar colonies were gray to white, opaque, smooth, dry and 1-3 mm in diameter after 24 hours of incubation. With an inoculating loop, colonies could easily be slid across the agar surface, like hockey pucks, and could be stacked like disks. Colonies on chocolate agar were pinkish- brown.The isolates which later identified as M.catarrhalis were showed large kidney shaped Gram negative cocci. Three (3.3%) isolates were positive for oxidase, catalase, DNase, tributary tests and reduce nitrate to nitrite, thus these strains were identified as M. catarrhalis, they were isolated from children aged 1 – 5years , 1 (30.3%) from female and 2 (60.6%) from male (Table 1).
|
|
4. Discussion
- In this study, 3 (3.3%) of isolates were identified asM.catarrhalisbased on colony morphology, Gram-stain and biochemical reactions. However, Ellis[16] reported that the phenomenon of colonies characters in conjunction with oxidase and catalase positivity and typical Gram-stain is the most settings appropriate for presumptive identification ofM.catarrhalis. Definitive identification should be differentiated it from the Neisseria. However, in the present study,M.catarrhalis was differentiating from Neisseria by its ability to grow into nutrient agar at 350C, hydrolysis of tributyrinand DNase. These results were almost similar to that reported by Broideset al.,[17] who found thatM.catarrhalis occurred in proportion of 4.8% in children less than 5 years and Vergison[18] who showed that M.catarrhalis proportion in children with otitis media was 3–20%.In this study M.catarrhalis was occurred most frequently in male than female and this was in alignment with previous report whichsuggested that the males are more liable to be infected by M.catarrhalis[11].AllM.catarrhalis isolates in the present study were sensitive to amoxiclav, azithromycin, ceftazidime, ceftriaxone, cephalexin, cefotaxime, chloramphenicol, ciprofloxacin, co- trimoxazole and erythromycin and showed resistant to ampicillin which might be due to its ability to production of β-lactamase enzyme. According to McGregor et al.,[19] mostM.catarrhalis isolates produce β-lactamases and resistant to penicillins; and susceptible to most other antibiotics, including erythromycin, tetracycline, co- trimoxazole, and the combination of penicillins with a β-lactamase inhibitor (e.g., clavulanic acid). However, it has been suggested that the production of β -lactamases by M.catarrhaliscould protect colonizing pathogens from the effects of β -lactam antibiotic treatment[10]. More than 90% of M catarrhalis strains have been shown to resist amoxicillin, and these rates vary by region. Amoxicillin-clavulanate, second- and third-generation oral cephalosporins, and trimethoprim-sulfamethoxazole(TMP-SMX) are the most recommended agents. Alternatively, azithromycin, clarithromycin, or dirithromycin can be used[11].In any case, the financial impact on global health care systems of the high incidence of M. catarrhaliscolonization and disease is significant, and consequently, several research groups are currently involved in identifying and assessing the usefulness of putative M. catarrhalisvaccine candidates[10].
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML