-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Microbiology Research
p-ISSN: 2166-5885 e-ISSN: 2166-5931
2013; 3(2): 71-78
doi:10.5923/j.microbiology.20130302.02
New Face in the Row of Human Therapeutics: Bacteriocins
Samir Giri, Jitendra Singh
School of Biotechnology, Gautam Buddha University, Greater Noida, 201308, Uttar Pradesh, India
Correspondence to: Jitendra Singh, School of Biotechnology, Gautam Buddha University, Greater Noida, 201308, Uttar Pradesh, India.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Bacteriocins, are large and functionally diverse family of antimicrobials found in all major lineages of bacteria. Recent studies reveal that these proteinaceous toxins play a significant role in mediating competitive dynamics between bacterial strains and closely related species. The capability of bacteria to effectively outcompete undesired species is often due to, or enhanced by, the production of potent antimicrobial toxins. In this review potential uses of bacteriocins and bacteriocin-producings strains as probiotics, in gastrointestinal tract infection, systemic infection, oral and respiratory tract infection, as anti-neoplastic agent as well as spermicidal and contraceptive agent has been addressed.
Keywords: Bacteriocins, Probiotics, Gastrointestinal tract infection, Systemic infection, Contraceptive, Anti-neopalstic agent
Cite this paper: Samir Giri, Jitendra Singh, New Face in the Row of Human Therapeutics: Bacteriocins, Journal of Microbiology Research, Vol. 3 No. 2, 2013, pp. 71-78. doi: 10.5923/j.microbiology.20130302.02.
Article Outline
1. Introduction
- Bacteriocins were initially identified about 100 years ago as a heat-labile product present in cultures of Escherichia coli V and toxic to E. coli S. They were given the name of colicin to identify the producing species[1]. Fredericq confirmed that colicins were proteins that had a limited range of activity owing to the presence or absence of specific receptors on the surface of sensitive cells[2]. Since then, bacteriocins have been found in all major lineages of bacteria and recently their production has been described by some members of the archaea[3, 4, 5]. According to Klaenhammer, 99% of all bacteria possibly will make at least one bacteriocin [6].Bacteriocins are bacterially produced natural peptides released by different varieties of bacteria and archea that are active against other bacteria and the producer has a specific immunity mechanism[6, 7]. This mechanism provides a competitive advantage in the environment remove competitors and very much helpful to gain resources in a better manner. Bacteriocins are ribosomally synthesized peptides and some of them are extensively post-translationallymodified. A large amount of bacteriocins have a relatively narrow spectrum of antimicrobial activity, i.e., inhibit growth of only certain species, generally those phylogenetically related to the producer strain. On the other hand, some bacteriocins exhibit a much broader spectrum of antimicrobial activity and may extend beyond the borders of bacteria to include protozoa, yeast, fungi, and viruses. A few bacteriocins are cytotoxic, with activity against sperm and tumorcells[8].Due to the clear ubiquity of bacteriocin production in different bacteria and archea implies its important role in bacterial survival. Bacteriocin possibly will function as colonizing peptides support the introduction or dominance of a producer into an already occupied place. Along with that bacteriocin also work as antimicrobial or killing peptides directly preventing competing strains or pathogens. Lastly bacteriocin may work as signaling peptides either signaling other bacteria through extracellular diffusible signaling molecules (quorum sensing) like in gram negative bacteria N(acyl) homoserine lactone typically serves as a signal molecule, whereas, in gram positive bacteria peptides including some bacteriocins and serve as a signaling agent. In addition of regulating their own synthesis, bacteriocins may also engage in interspecies communication or bacterial cross talk within microbial diversity or recently impact of bacteriocins on signaling cells of host immune system[9, 10, 11, 12, 13, 14].Bacteriocins are distinguished from classical antibiotics by two main features: bacteriocins are ribosomally synthesized and have a relatively narrow killing spectrum [4]. The bacteriocin family includes a variety of proteins in terms of size, mode of action, microbial target, release, and immunity mechanisms and can be divided into two major groups: those produced by Gram-negative bacteria and Gram-positive bacteria[15, 16].
2. Sources
2.1. Bacteriocins from Different Gram-Negative Bacteria
- Recent surveys of E. coli, Salmonella enterica, Hafniaalvei, Citrobacterfreundii, Klebsiellaoxytoca, Klebsiellapneumoniae, and Enterobacter cloacae revealed a certain level of production of bacteriocin from 3-26% of environmental isolates[15, 17]. Colicins, bacteriocins produced by E. coli, are found in 30–50% of the strains isolated from human hosts and are often referred to as virulence factors[18].Some Gram-negative bacteria such as Pseudomonas aeruginosa, show much higher levels of bacteriocin production, in which>90% of both environmental and clinical isolates produce bacteriocins[19].The colicins of E. coli are the most extensively studied gram-negative bacteriocins, since the time of their discovery. They now serve as a model system for investigating the mechanisms of bacteriocin structure / function, genetic organization, evolution and ecology[20]. Colicins are high molecular weight proteins that can kill target cells by a variety of mechanisms. Colicins are usually encoded on one of two types of colicinogenicplasmids[21]. Type A plasmids are small (6 to 10 kb) and present in numerous copies per cell. They are mobile in the presence of a conjugative plasmid and are amplifiable. Type B are monocopy plasmids of about 40 kb, and carry numerous genes, in addition to that encoding colicin activity and have the ability to conjugate. A close relative to the colicins, the bacteriocins of Serratiamarcesens, is found on both plasmids and the chromosome[22, 23].A colicin protein consists of three functionally distinct domains: receptor recognition, protein translocation, and killing domain[24]. Besides colicins, E. coli strains and gram-positive bacteria produces a second type of bacteriocin, known as microcins, which are smaller than colicins and have similar properties to bacteriocins, including thermostability, resistance to some proteases, relative hydrophobicity, and resistance to extreme pH[25, 26, 27]. Till date fourteen microcins have been reported, of which only seven have been isolated and fully characterized.
2.2. Bacteriocinsfrom different Gram-Positive Bacteria
- Bacteriocins of gram-positive bacteria are evenly abundant and even more diverse than those found in gram-negative bacteria. The Gram-positive bacteriocins are similar to many of the antimicrobial peptides produced by eukaryotes; they are generally cationic, amphiphilic, membrane-permeable peptides, and range in size from 2-6 kDa[28]. They differ from bacteriocins of gram-negative bacteria in essentially two ways[4]. First, the bacteriocins produced by gram-positive bacteria are not necessarily lethal to the producing cell. This critical difference is due to dedicated transport mechanisms encoded by gram-positive bacteria to release the bacteriocin toxin. Normally, their biosynthesis is self-regulated with specifically dedicated transport mechanisms facilitating release. Second, the gram-positive bacteria have evolved bacteriocin-specific regulation whereas bacteriocins of gram-negative bacteria depend on host regulatory networks[29].Gram-positive bacteriocins, in common, and lantibiotics, in particular, require several more genes for their production than do those of gram-negative bacteria[30]. The nisin gene cluster, for example, includes genes for the prepeptide (nisA), enzymes for modifying amino acids (nisB, nisC), cleavage of the leader peptide (nisP), secretion (nisT), immunity (nisI, nisFEG), and regulation of expression (nisR, nisK). These gene clusters are most often encoded on plasmids but are infrequently found on the chromosome[31]. Several gram-positive bacteriocins, including nisin, are located on transposons[32].Production of bacteriocins in gram-positive bacteria is generally associated with the shift from log phase to stationary phase. For example, nisin production begins during mid-log phase and increases to a maximum as the cells enter stationary phase[33]. The regulation of expression is not cell cycle dependent, per cell, but rather culture density dependent[34].
3. Therapeutic Applications of Bacteriocins
- These days everyone expects to see bacteriocins related to food applications, their many promising uses for the control of undesired microorganisms in the human environment are seriously appreciated. With the advent of multidrug resistant bacteria, it has become a priority to develop alternative medicinal treatments preventive measures against these pathogens. Since the mode of action of bacteriocins is remarkably different from conventional antibiotics, they may be considered as a novel source for the control of microbial pathogens.
3.1. Role of Bacteriocins as Probiotic
- The term “probiotic,” which literally means “for life,” has since been employed to describe these health-promoting bacteria. The World Health Organization has defined probiotic bacteria as “live microorganisms which when administrated in adequate amounts confer a health benefit on the host”[35]. Probiotic bacteria (PB) have been historically used to treat a variety of ailments, including infections of mucosal surfaces such as the vagina and the gastrointestinal (GI) tract. However, with the discovery and development of antibiotics in the twentieth century, the perceived value of these traditional therapies diminished. Today, with the decline in efficacy of antibiotics and a dramatic resurgence of infectious disease, physicians, researchers, and the public are reconsidering the possible role of probiotics as an alternative to supplement existing antibiotic dominated therapies[36, 37]. Over the past 15 years, there has been an increase in research on probiotic bacteria and a rapidly growing commercial interest in the use of probiotic bacteria in food, medicine, and as supplements[38, 39]. Conventionally, for the selection of probiotic strains, production of bacteriocin has been considered an important characteristic. In recent time, some of the studies have verified the ability of a strain to compete within complex microbial diversity during the impact of bacteriocin production which is also positively involved in the health of the host. Bacteriocins directly prevent the attack of competing pathogens or adjust the composition of microbiota and influence the immune system of host[40].A number of probiotic bacteria have been targeted as potential therapeutic agents. Examples include lactic acid bacteria (LAB), Bifidobacteria[41], Saccharomyces[42], Enterics[43], and Streptococci[44]. Potential PB species differ in terms of their bioavailability, metabolic activity, and mode of action. However, to be used in host-associated activities, they all must be non-pathogenic and non-toxic. Antimicrobial activity is thought to be an important means for PB to competitively exclude or inhibit invading bacteria[40, 45]. Some do so by secreting non- specific antimicrobial substances, such as short-chain fatty acids[40] or hydrogen peroxide[46], while others produce toxins with very narrow killing ranges, such as bacteriocins, bacteriocin-like inhibitory substances (BLIS), and bacteriophages[47, 48].The food industry utilizes bacteriocin to reduce the use of chemical preservatives in foods which have a limited shelf life, or those foodstuffs that present a high risk for pathogen contamination. The best known examples of biopreservation involve bacteriocins[49].
3.2. Role of Bacteriocins in Gastrointestinal Tract
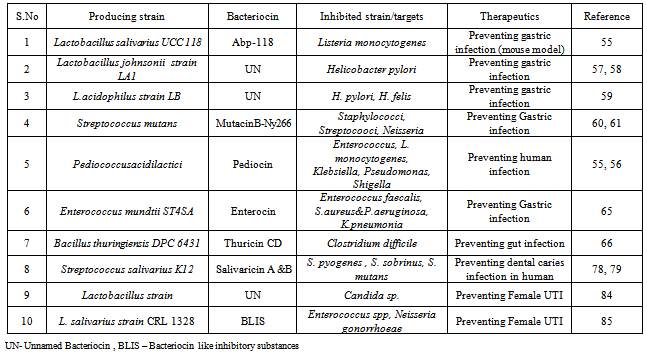
- Gastrointestinal tract of human is a complex ecosystem in which a delicate equilibrium exists between the intestinal microflora and the host. The microflora [LAB, lactic acid bacteria] play an important role as a major stimulus for the development of the mucosal immune system[50, 51].Two main genera of lactic acid bacteria dominate the intestinal flora, together with 56 species of Lactobacillus and several species of Bifidobacterium. Most of these species have been shown to produce bacteriocinsin vitro[52, 53, 54]. Lactobacillus salivariusstrain UCC118, which produces an effective broad-spectrum bacteriocin (Abp118) active against the food-borne pathogen Listeria monocytogenes. In mice, the L. salivariusstrain provided protection against L.monocytogenesinfection, while a mutant strain of the same species, impaired in its bacteriocin production as well as protection ability [55].The release of bacteriocins inhibiting Helicobacter pylori, a human pathogen that causes severe gastroduodenal diseases[56], has been predominantly studied in lactobacilli strains. A bacteriocin like inhibitory substance (BLIS) with anti-H.pyloriactivity was acknowledged in probiotic Lactobacillus johnsoniistrain LA1[57,58] and Lactobacillus acidophilus strain LB[59]. Mutacin B-Ny266, a lantibiotic formed by Streptococcus mutans, inhibit a broad spectrum of multi-resistant pathogens including Staphylococci, Streptococci, and Neisseria strains[60, 61].Pediocin from Pediococcusacidilactici has a fairly broad inhibitory spectrum and can inhibit Streptococcus aureus, Bacillus spp. Listeria and vegetative cells of Clostridium spp.[62, 63, 64]. A different promising probiont is the bacteriocin producer Enterococcus mundtiistrain ST4SA, active against a number of gram-positive bacteria, including Enterococcus faecalis, Streptococcus pneumoniae, and Staphylococcus aureus, in addition to the gram-negative bacteria P. aeruginosaand K. pneumonia[65].Thuricin CD from Bacillus thuringeinsis DPC 6431 particularly eliminates Clostridium difficilebacteria from among the trillions of bacteria in a model gut system[66]. Researchers are beginning to classify microbes and human microbial populations that are associated with conditions as wide-ranging as liver, coeliac and inflammatory bowel diseases, obesity, diabetes, irritable bowel syndrome, colon cancer, pouchitis and even mental health. Researchers believe that bacteriocins could be used in the future to design and shape ‘healthy’ bacterial communities. The specific mechanisms by which probiotic bacteria inhibit pathogens such as C. difficile are as yet poorly understood. Recent work revealed that the therapeutic potential of the probiotic strain Lactobacillus salivarius is due, at least in part, to its ability to produce a potent two-peptide bacteriocin, Abp118[67]. In addition, recently showed significant anti-C. difficile potential for yet another bacteriocin, the two-component lantibioticlacticin 3147, produced by Lactococcuslactis. Significantly, and in contrast to conventional broad-spectrum antibiotics, lacticin 3147 completely eliminates 106 c.f.u. C. difficile per ml within 30 min (at concentrations as low as 18 mg per ml) without considerably impacting on the normal resident microflora[68]. While this effort involved in vitro studies in model faecal environments, in vivo sensitivity of the bacteriocin to gastric acidity creates a technological / delivery hurdle which will have to be overcome if this bacteriocin is to achieve its potential as an effective oral therapeutic.While extensive advancement has been made with respect to our understanding of bacteriocin structure / function, regulation, and immunity, further study is essential to expand a complete understanding of the factors which control bacteriocin production in the GI tract because bacteriocin actively produced in vitro may not necessarily be produced in sufficiently high quantities, or at all, with in the GI tract[40].
3.3. Role of Bacteriocins in Systemic Infection
- Systemic infection is one of the most challenging condition in number of disease like HIV, hypertension, atherosclerosis, diabetes mellitus etc. and number of associated bacteria those are mostly involved in the systemic infection like S. aureus, Listeria monocytogenes, and P. aeruginosa. Number of bacteriocin present that play an important role to inhibit the growth of the bacteria like nisin inhibited the growth of P. aeruginosawhen used in the combination with polymyxin E and clarithromycin antibiotics[69]. Nisin and lacticins A164 and BH5 inhibited the growth of H. pylori in vitro and may be used in the treatment of peptic ulcers[70]. Bacteriocin like lantibioticsduramycin, duramycin B and C and cinnamycin inhibit phospholipase A2 indirectly by sequestering the substrate phosphatidylethanolamine and may be used as an anti inflammatorydrug[71]. Phospholipase A2 participate in arachidonic acid cascade leading to the production of potent mediators of inflammation and allergy including the prostaglandins, leukotrienes and hydroxyeicosatetraenoicacids[72, 73]. Cinnamycin like lantibiotics and ancovenin, a type B lantibiotics, inhibit the activity of the angiotensin converting enzyme (ACE). These angiotensin converting enzyme catalyzes the conversion of angiotensin I to angiotensin II and degrade bradykinin in that way regulate the blood pressure and fluid balance[74, 75]. So bacteriocin may thus have potential for treating high blood pressure.
3.4. Role of Bacteriocins in Oral cavity and Respiratory tract
- Streptococci, in particular, S. mutansand Streptococcus salivarius, are considered the principal etiological agents of dental caries in humans[76,77]. S.mutansproduces mutacins active against neighboring plaque-forming strains, and a positive relationship exists between bacteriocin production and the ability to colonize the oral cavity. A nonpathogenicmutacin producing strain was constructed for use in dental caries replacement therapy[76].S. salivariusK12 produces two potent lantibiotics, salivaricin types A and B. This strain is employed to treat infections of the upper respiratory tract caused by streptococcal organisms, including treatment of dental caries caused by S. sobrinusand S. mutans[78]. Streptococcus pyogenesis a common human commensal, with 5–15% of the human population harboring the bacterium, usually in the respiratory tract, without signs of disease. However, strains of S. pyogenescan become pathogenic when host defenses are compromised[79]. S. salivarius, found to produce bacteriocins with anti-S. pyogenesactivity. In the lab, this bacteriocin was capable to kill a range of other human pathogens, including Moraxelacatarrhalisand Haemophillusinfluenza[80].
3.5. Role of Bacteriocins in Female UTI
- The healthy human vaginal microbiota is dominated by Lactobacillus crispatus, Lactobacillus jensenii,Lactobacillus iners, and Lactobacillus gasseri[81]. In contrast, thevaginal microbiota of women with bacterial vaginosis is dominated by Gardnerellavaginalis, Mycoplasma hominis, Prevotella, Peptostreptococcus, Mobiluncusspp., andBacteroidesspp., while lactobacilli are found at lower densities[82, 83]. Bacteriocin production by probiotic lactobacilli strains was found to inhibit the growth of some of these infectious pathogens: L. acidophilus and L. jenseniistrain 5L08 showed antagonistic activity against G. vaginalis. L. pentosusstrain NCIMB 41114 was patented for its use as a probiotic agent because it competitively excludes various species of Candida[84]. Most promising vaginal probiont so far is a vaginal isolate of L. salivariusstrain CRL 1328, found to release a BLIS able to inhibit the growth of certain strains of Enterococcus spp., as well as Neisseria gonorrhoeae[85].
|
3.6. Antineoplastic Activity of Bacteriocins
- Bacteriocins are antimicrobial peptides also show antineoplastic activity which have been inadequately revealed in the late 70s by using crude bacteriocin preparation toxic to mammalian cells. These days, purified bacteriocins are available and have shown inhibitory properties toward various neoplastic celllines. Pyocin, colicin, pediocin, and microcin are among bacteriocins reported to present such activity. Besides that, custom-made bacteriocins proved to be effective in a glioblastomaxenograft mouse model. Bacteriocins have also been suggested as a cancer treatment. But their status as a form therapeutic agent remains experimental[86].
3.7. Bacteriocins in Contraceptive and Spermicidal Activity
- Some of the bacteriocins that are active against vaginal pathogens are also reported as having spermicidal activity. This feature makes them attractive for formulation in feminine health care and contraceptive products. In order to evaluate nisin’s spermicidal activity,Aranha et al, developed a contraceptive model in rats[87]. Nisin, dissolved in saline, was administered into the vagina of the animals for 14 consecutive days. Animals were then immediately allowed to mate, and none of the nisin treated animals became pregnant. No histopathological lesions were observed in the vaginal epithelium, and liver and kidney function remained normal. Fertility was also restored after experiments. According to the authors, 1 mg of nisin was able to completely halter sperm motility[87]. This is an interesting finding, since many commonly used contraceptive products contain Nonoxynol-9 (N-9), a compound harmful to epithelium. But if the concentrations of nisin used in the animal model are extrapolated for human usage, they are well above the limits of what the healthy vaginal microflora can survive. Thus, nisin cannot be practically considered for use in human[88]. Subtilosin, a bacteriocin produced by B. amyloliquefaciens,was also shown to have potent spermicidal activity[89].
4. Conclusions
- Bacteriocins are antagonistic to many important human pathogens. Bacteriocins have the ability to target a relatively narrow range of bacteria without affecting much of the natural microbiota of the body, which is an important advantage, especially when compared to other antibiotics. The application of bacteriocins as therapeutic agents is a rapidly developing areatherefore, they work like another tool to combat infections especially important with consideration of the ever-growing problem of antibiotic resistance. Some of bacteriocins and their targets are summarized in Table 1, and in future there is still much to investigate in this field.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML