-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Microbiology Research
p-ISSN: 2166-5885 e-ISSN: 2166-5931
2013; 3(2): 67-70
doi:10.5923/j.microbiology.20130302.01
Microbiological Quality of Water in Fulani Settlements in Gidan Kwano, Minna, Niger State, Nigeria
Eseoghene O. Egbe1, John D. Mawak2, Oluwafemi A. Oyewole1
1Department of Microbiology, Federal University of Technology, Minna, Niger State, Nigeria
2Department of Microbiology, University of Jos, Jos, Nigeria
Correspondence to: Eseoghene O. Egbe, Department of Microbiology, Federal University of Technology, Minna, Niger State, Nigeria.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
The study was conducted to determine the microbiological quality of drinking water sources in Fulani settlements in GidanKwano, Minna, Niger state. Thirty water samples were collected from streams, wells and taps and examined for Total coliform and Total viable counts (TVC) using the multiple tube fermentation tests and the pour plate technique respectively. Isolates were identified using standard biochemical tests. All the water sources were found to contain coliforms in numbers exceeding the SON standards for water. The total viable counts for all the water sources also exceeded the limit; 100 cfu/ml for water. Most Probable Number of coliforms ranged from 3 MPN/100ml to 1100 MPN/100ml. All water sources were contaminated the following bacterial pathogens Citrobacterdiversus, Citrobacterfreundii, Klebsiellapnuemoniae, Proteus vulgaris, Salmonella enteric and Serratiamarcescens. The study revealed a contamination of all water sources in the study area. It is therefore suggested that Hygiene practices should be improved so as to reduce contamination by microbial flora.
Keywords: Microbiological Quality, Water, Fulani Settlements
Cite this paper: Eseoghene O. Egbe, John D. Mawak, Oluwafemi A. Oyewole, Microbiological Quality of Water in Fulani Settlements in Gidan Kwano, Minna, Niger State, Nigeria, Journal of Microbiology Research, Vol. 3 No. 2, 2013, pp. 67-70. doi: 10.5923/j.microbiology.20130302.01.
Article Outline
1. Introduction
- The microbiological quality of water used for human consumption is crucial as it influences human health. It can be contaminated by a wide variety of microorganisms some of which are pathogenic. Contamination of drinking water with human or animal excreta is frequently associated with diseases in man. The occurrence of such diseases can be reduced through efficient water treatment processes but water analysis is essential; particularly in the absence of such processes. International standards for water quality are targeted at ensuring the absence of pathogenic microorganisms in drinking water because water contamination with pathogenic microorganisms has been commonly associated with the transmission of infectious diseases that have caused serious illnesses and associated mortality worldwide[1].The essential parameters recommended by WHO for the monitoring of water supplies are: Escherichia coli and thermo-tolerant coliforms accepted as suitable substitutes, Chlorine residual (if chlorination is practiced), pH and Turbidity [2]. Escherichia coli and thermo-tolerant coliforms are used because these organisms are indicative of faecal pollution. As such, they are referred to as indicator organisms. They are comparatively easy to isolate and enumerate and, their presence infers that pathogens may also be present. Furthermore, colony counts of these organisms can also give an indication of the overall microbiological quality of the water.The microbiological assessment of a particular water resource provides up to date information on the quality and safety of the water; hence, it is necessary to perform microbial assessment of water sources regularly to ensure continued safety of water supply within communities. This study is aimed at determining the microbiological quality of water in Fulani settlements in GidanKwano, Minna, Niger State using indicators of pollution such as total coliforms and thermo-tolerant coliforms.
2. Materials and Method
- Although none of the wells had the depth of a standard deep well (30m), for the purpose of this study, the well water samples were categorized into two based on depth as shallow wells and deep wells representing wells with 0-2m depth and 9.5-10.5m depth respectively.
2.1. Sample Collection
- A total of 30 samples from drinking water resources were collected for a period of 8 weeks (July to August, 2011). Water samples for analysis were collected in closed sterilized glass containers (200 ml volume) aseptically, transported to the laboratory on ice, kept at low temperature and analysed. The sampling bottles were legibly labelled. Well water samples were collected into sterile bottles tied with a strong twine to a piece of metal as the weight. The bottles were aseptically opened and lowered into the well. When filled, the bottles were gently raised to the surface and covered. For collection of stream water samples, water was collected aseptically midstream and covered immediately. For collection of borehole water samples, the nozzles were properly cleaned and the water left to run for about 2-3 minutes to ensure flushing of stagnant impurities in the pipe. A piece of cotton wool soaked in methylated spirit was ignited and used to heat-up the tap nozzle until it became unbearably hot to touch to avoid external contamination. The water was then allowed to run continuously for about 1 minute to cool the water after which the sample bottles were filled with water and covered carefully.Microbiological analyses of water samples were performed as defined by the Nigerian Standards for Drinking Water Quality[3]. Total coliform and Escherichia coli were determined by means of the multiple tube fermentation tests[4]. The total viable counts (TVC) in the water samples were obtained using the pour plate technique. Biochemical tests were used to isolate and characterize the microorganisms present in the water.
2.2. Estimation and Isolation of Total Coliforms and Escherichia Coli
- Microbial quality of the drinking water samples was determined using the multiple tubes fermentation test[4]. The 3-tube most probable number (MPN) method was used to estimate Total coliforms. Presumptive test was carried out using inoculated tubes of Lactose broth incubated at 37°C for 48 h. Positive presumptive tests were confirmed using Eosin methylene blue agar plates incubated at 37ºC for 24 hours.
2.3.Isolation of Salmonella and Shigella
- Salmonella spp. and Shigella spp. were isolated using Salmonella-Shigellaagar followed by sub culturing of non-lactose fermenting colonies on nutrient agar. All the inoculated media were incubated at 37ºC for 24 hours. Isolates were then identified using colonial characteristics on media as well as standard biochemical reactions.
2.4.Total Viable Counts (TVC)
- The total viable counts (TVC) in the water samples were obtained using the pour plate technique[5]. Dilutions of water samples in peptone saline solution were inoculated in 1 ml aliquots into each of 15 ml molten nutrient agar in sterile petri dishes and incubated at 37°C for 24 hours. Petri dishes from dilutions containing between 25 and 250 discrete colonies were counted and the results expressed as the numbers of bacteria colonies per millilitre of the sample.
2.5. Biochemical Tests
- Isolates were subjected to a number of tests to identify the organisms present using described methods[6-7]. These biochemical tests include; Gram staining, indole, citrate utilization, Carbohydrate fermentation, hydrogen sulphide production, motility and urease.
2.6. Statistical Analysis
- The total coliforms and total viable plate counts of the various water sources were evaluated using the statistical program for the social sciences (SPSS) version 16. The average coliform bacteria per 100 ml and average total heterotrophic bacteria plate counts per ml were used to evaluate the microbial quality of water in this study.
3. Results and Discussions
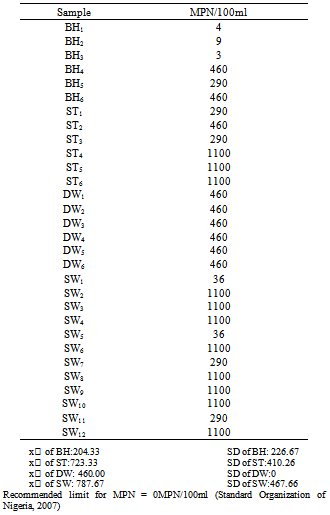
- Water from the various sources were assigned different codes; BH was used to represent borehole water, ST was used to represent water drawn from streams, DW was used to represent water drawn from deep wells and SW was used to represent water drawn from shallow wells. The various samples of the water sources were identified using subscript numbers. Also, for the purpose of the study, mean (x̅) and Standard Deviation were calculated and used for the analysis of the result.
|
|
4. Conclusions
- The total viable count and most probable number of coliforms in all the water sources did not comply with the standards. Contamination with primary fecal indicator Escherichia coli was not observed in any of the water sources. The microbiological quality of water sources in Fulani settlements in GidanKwano can be said to be low due to their inability to meet standards. It is therefore recommended that; hygiene practices should be improved so as to reduce contamination by microbial flora, Protective covering should be provided for all the wells to reduce external-source contamination and clean containers should be used for drawing water from the wells.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML