-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Microbiology Research
p-ISSN: 2166-5885 e-ISSN: 2166-5931
2013; 3(1): 39-42
doi:10.5923/j.microbiology.20130301.06
Prevalence of Methicillin–Resistant Staphylococcus aureus (MRSA) in the Community of Al-Majmaah/Saudi Arabia and Possibility of Resistance to Vancomycin and other Antimicrobial Agents
Khalil Y. Abujheisha
Department of Medical Laboratories, College of Applied Medical Science, Al-Majmaah University, 11932, Saudi Arabia
Correspondence to: Khalil Y. Abujheisha , Department of Medical Laboratories, College of Applied Medical Science, Al-Majmaah University, 11932, Saudi Arabia.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
To determine the prevalence of methicillin resistant Staphylococcus aureus (MRSA) strains among clinical isolates collected from 2 tertiary hospitals in Al- Majmaah, Saudi Arabia and to test the possibility of resistance to vancomycin and other antimicrobial agents. A total of 106 S. aureus clinical isolates were collected during a period of 6 months. The sensitivity patterns of these isolates were determined using the Kirby-Bauer disc diffusion method. The prevalence of MRSA among S. aureus isolates was (43.4%) 46/106. Among 46 MRSA isolates, 82.61% showed multidrug resistance to Ciprofloxacin Tetracycline, Chloramphenicol, Kanamycin and Erythromycin. 82.6 % of MRSA were sensitive to gentamicin. No resistance to Vancomycin. The rate of MRSA resistance in this study was higher than what had been reported in other areas of Saudi Arabia and other countries and the majority shows multidrug resistance .
Keywords: Methicillin, Staphylococcus Aureus, Resistance , Vancomycin, Antimicrobial Agents
Cite this paper: Khalil Y. Abujheisha , Prevalence of Methicillin–Resistant Staphylococcus aureus (MRSA) in the Community of Al-Majmaah/Saudi Arabia and Possibility of Resistance to Vancomycin and other Antimicrobial Agents, Journal of Microbiology Research, Vol. 3 No. 1, 2013, pp. 39-42. doi: 10.5923/j.microbiology.20130301.06.
Article Outline
1. Introduction
- Staphylococcus aureus is a major pathogen associated with serious community and hospital-acquired diseases associated with high morbidity and mortality worldwide. The rapid evolution of antibiotic resistance in S. aureus is of considerable concern. Methicillin was introduced in 1959 to treat infections caused by penicillin-resistant Staphylococcus aureus. In 1961 the first S. aureus isolates that had acquired resistance to methicillin (methicillin-resistant S. aureus, MRSA) were reported from the United Kingdom[1] and were soon recovered from other European countries, and later from Japan, Australia, and the United States. MRSA is a major pathogen in hospitals worldwide and has become gradually more difficult to treat due to increasing resistance[2,3]. There is a wide range in the prevalence of MRSA strains between different countries and even between hospitals in the same country. The extent of the spread of these organisms from hospital to hospital also shows variation[4]. There are several mechanisms responsible for methicillin resistance. The most important is the production of the penicillin-binding protein PBP2a encoded by the mecA gene. In addition, hyperproduction of β –lactamase and modified drug affinities of the usual PBPs are considered as minor resistant mechanisms[4,10,11].Vancomycin and teicoplanin are glycopeptides with significant activity against gram positive bacterial pathogens [12] and over the past two decades, vancomycin has been considered the drug of choice for MRSA infections. Unfortunately, in 1996, the emergence of vancomycin-resistant S. aureus (VRSA) in Japan and thereafter from USA and other countries has caused additional concern[14,15]. Therefore, infections caused by these resistant strains are very serious and difficult to treat.The aim of this study was to determine the prevalence of MRSA strains isolated from nosocomial infections and their antimicrobial resistant patterns including vancomycin in 2 tertiary hospitals in Majmaah, Saudi Arabia.
2. Materials and Methods
2.1. S. aureus Isolates
- From October 2011 – April 2012, a total of 106 non replicate S. aureus clinical isolates from hospitalized patients were collected from 2 tertiary hospitals in the community of Almajmaah (Table 1). Infections were defined as hospital acquired when the patient had been hospitalized for more than 48 h[16]. The identity of these isolates was confirmed using colonial morphology on blood agar plates (Oxoid, UK), growth and fermentation of manitol in Manitol Salt agar (BBL TM), gram stain and positive catalase and coagulase tests (Murex Diagnostic Ltd., UK).
2.2. Antibiotic Susceptibility Testing
- Susceptibility testing was performed using Kirby-Bauer disk diffusion method on Mueller-Hinton agar plates (Oxoid) as recommended by the Clinical LaboratoryStandards Institute (CLSI), formerly NCCLS[17]. Briefly, the tests were performed by diluting colonies of S. aureus grown overnight on sheep blood agar (Oxoid) in normal saline equivalent in density to 0.5 McFarland barium sulfate standard unit. The entire surface of the Mueller-Hinton agar plate was covered with the required inoculum, and the plates were allowed to dry for 5 minutes before the antibiotic discs were placed on the surface and incubated for 18–24 h at 37℃. Following the incubation period, the sensitivity results were determined by comparing the diameter of the zones of inhibition with CLSI standards[17]. The antibiotics tested (Oxoid) were Pencillin 10µg, Oxacillin 1 µg, Ampicillin 10µg, Ciprofloxacin 5µg, Tetracycline 30µg, Chloramphenicol 30µg, Kanamycin 30µg, Erythromycin 15µg, Gentamycin 10µg, Amoxicillin/clavulanic acid 30µg and Vancomycin 30µg. S. aureus ATCC 25923 was used as a control strain.
2.3. MRSA Testing
- Isolates were tested for methicillin resistance by the Kirby-Bauer disc diffusion method as described above using an oxacillin disc (1µg) on Mueller-Hinton agar supplemented with 4% NaCl and incubated at 35° C for 24 h. A zone of inhibition of ≥13 mm was considered as oxacillin sensitive[17]. Methicillin resistance results were confirmed by pencillin binding protein PBP2 –Latex agglutination test (Oxoid, UK) which is based on the agglutination of latex particles sensitized with monoclonal antibodies against PBP2a.
2.4. Vancomycin Resistance Testing
- MRSA were tested for vancomycin resistance by Kirby-Bauer disc diffusion method as described above using vancomycin disc (30µg). A zone of inhibition of ≤14 mm was considered as vancomycin resistant[17]. Multidrug resistance was defined as resistance to penicillin and oxacillin plus 3 or more of the following agents: Erythromycin, Ciprofloxacin, Kanamycin and Tetracycline [18].
3. Results
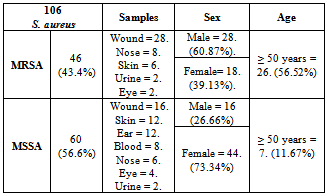
- Of the 106 S. aureus strains tested, 46 (43.4%) contained PBP2a and were methicillin resistant according to the disc diffusion test. Similar results were found with the latex agglutination test which further confirmed MRSA results. Rates of resistance of methicillin-sensitive S. aureus(MSSA) and MRSA to the other antibiotics tested are shown in (Table 3). Of 46 MRSA isolates, 82.61% were multidrug resistant.Infections with MRSA were more common among males (60.87%) and older (≥ 50 years) than females and younger patients. Among the 46 MRSA strains, 28 (60.87%) were isolated from wounds, 8 (17.4%) from nose, 6 (13.04%) from skin abscess, 2 (4.35%) from urine and 2 (4.35%) from eye. While the 60 MSSA strains, Wound = 16 (26.67%), Skin = 12 (20%), Ear = 12 (20%), Blood = 8 (13.33%), Nose = 6 (10%), Eye = 4(6.67%), Urine = 2 (3.33%). (Table 2).
|
|
4. Discussion
- In the United States and in some European countries, MRSA accounts for 10 to 40% of all S. aureus isolates. In Spain, the prevalence of MRSA has increased from 1.5% in 1986 to 31.2% in 2002[5,6]. In Algeria, the rate increased from 10% in 1997 to 14% in 2001[7]. In Saudi Arabia hospitals ranged from 12% to 49.4% in Riyadh and 38.9% in Makkah hospitals without any report of resistance to vancomycin[8,9]. In this study, the rate of resistance (43.4%) of S. aureus is much higher than what has been reported by researchers in other regions of Saudi Arabia as well as in some other Studies[8,9,19]. Microbes have genetic plasticity, which means that they have the capacity to evolve in response to their environment. The major impetus for developing resistance is selective pressure resulting from antibiotic use. The bacteria that survive are those that develop mechanisms to avoid being killed by antibiotics. Although new antibiotics can effectively treat some resistant pathogens, bacteria will eventually develop resistance to any antibiotic with time. The misuse and overuse of antibiotics drive the emergence and spread of resistance.In Japan, the first strain of S. aureus with reduced susceptibility to vancomycin (VISA/VRSA) was isolated in 1996[14] and as of 2002, infections with such strains in was confirmed patients in USA[15]. Later on, the emergence of such cases has been confirmed in India[13]. hVISA isolated in some Asian countries like South Korea, Japan, Philippines, Singapore and Thailand[24,26]. All MRSA isolates in this study were sensitive to vancomycin and the majority sensitive to gentamicin, but the majority of our isolates (82.61%) showed multidrug resistance to Erythromycin, Ciprofloxacin, Kanamycin and Tetracycline.
5. Conclusions and Recommendations
- The rate of MRSA resistance in this study is much higher than what has been reported in other areas of Saudi Arabia and many other international countries. Keeping in mind the increasing prevalence rate of MRSA, it is extremely important to implement a revised strategy for MRSA isolates in hospitals and in community because any delay or wrong choice of antibiotics is avoidable and to improve treatment and control.
ACKNOWLEDGEMENTS
- The author gratefully acknowledge the help of Al-majmaah University (Deanship of scientific research) for the grant of this project and the help and support of my colleagues and students in Medical laboratories department.
References
| [1] | Jevons, M. P. Br. Med. J. 1, 124–125. 1961. |
| [2] | Hussain, F. M., Boyle-Vavra, S., Bethel, C. D. & Daum, R. S. Pediatr. Infect. Dis. J. 19, 1163–1166. 2000. |
| [3] | Centers for Disease Control and Prevention Morbid. Mortal. Wkly. Rep. 48, 707–710. 1999. |
| [4] | Struelens M, Mertens R, Groupement P. National survey of methicillin-resistant Staphylococcus aureus in Belgian hospital: detection methods, prevalence, trends and infection control measures. Eur J Clin Microbiol Infect Dis; 13: 56–63. 1994 |
| [5] | Bouza, E., J. Martinez-Beltran, and Grupo de Trabajo para el Estudio de Estafilococos. Estudio multice´ntrico sobre la prevalencia de estafilococos en Espan˜a. Enferm. Infecc. Microbiol. Clin.. 6:68–79. 1988. |
| [6] | Cuevas, O., E. Cercenado, A. Vindel, J. Guinea, M. Sanchez-Conde, M. Sanchez-Somolinos, and E. Bouza. Evolution of the antimicrobial resistance of Staphylococcus spp. in Spain: five prevalence studies, 1986 to 2002. Antimicrob. Agents Chemother.. 48:4240–4245. 2004. |
| [7] | Ramdani-Bouguessa N, Bes M, Meugnier H, Forey F, Reverdy ME, Lina G, Vandenesch F, Tazir M, Etienne J. Detection of Methicillin-Resistant Staphylococcus aureus Strains Resistant to Multiple Antibiotics and Carrying the Panton-Valentine Leukocidin Genes in an Algiers Hospital. Antimicrobial agents and chemotherapy, Vol. 50, No. 3. p. 1083–1085.2006. |
| [8] | Baddour M., Abuelkheir M. and Fatani J. Trends in antibiotic susceptibility patterns and epidemiology of MRSA isolates from several hospitals in Riyadh, Saudi Arabia. Annals of Clinical Microbiology and Antimicrobials, 5:30.2006. |
| [9] | Asghar H., Aiman M. Momenah. Methicillin Resistance among Staphylococcus aureus isolates from Saudi Hospitals. Med Princ Pract; 15:52–55.2006. |
| [10] | Duckworth G, Lothian J, Williams J. Methicillin-resistant Staphylococcus aureus : report of an outbreak in a London teaching hospital. J Hosp Infect;11: 1–5.1988. |
| [11] | Tomasz A, Drugeon H, de Lencastre H, Jabes D, McDougall L, Bille J. New mechanism for methicillin resistance in Staphylococcus aureus: clinical isolates that lack the PBP2a gene and contain normal penicillin-binding capacity. Antimicrob Agents Chemother; 33: 1869–1874. 1989. |
| [12] | Reynolds PE, Brown DF. Penicillin-binding proteins of β-lactam-resistant strains of Staphylococcus aureus. Effect of growth conditions. FEBS Lett; 192: 28–32.1985. |
| [13] | H. Krishna and M. Ranjan Sen. Emergence of vancomycin resistant Staphylococcus aureus (VRSA) from a tertiary care hospital from northern part of India. BMC Infectious Diseases, 6:156.2006. |
| [14] | Hiramatsu K, Hanaki H, Ino T et al. Methicillin-resistant Staphylococcus aureus clinical strain with reduced vancomycin susceptibility. J Antimicrob Chemother; 40: 135–6.1997. |
| [15] | T. R. WALSH, et al. Evaluation of Current Methods for Detection of Staphylococci with Reduced Susceptibility to Glycopeptides. Journal of clinical microbiology, p. 2439–2444.2001. |
| [16] | Blandino G, Marchese A, Ardito F, Fadda G, Fontana R, Lo Cascio G, Marchetti F, Schito G, Nicoletti G. Antimicrobial susceptibility profiles of Pseudomonas aeruginosa and Staphylococcus aureus isolated in Italy from patients With hospital-acquired infections. Int J Antimicrob Agents; 24: 515–518.2004. |
| [17] | National Committee for Clinical Laboratory Standards. Performance standards for antimicrobial tests. NCCLS document M2-A8.Wayne, National Committee for Clinical Standards, 2002. |
| [18] | Bell J, Turnidge J, SENTRY APAC Participants. Antimicrobial resistance trends In community-acquired respiratory tract pathogens in the Western Pacific Region and South Africa: report from SENTRY antimicrobial surveillance program, 1998–1999, Including an in vitro evaluation of BMS284756. Int J Antimicrob Agents;19 125–132.2002. |
| [19] | Bukharie H, Abdelhadi M: The epidemiology of methicillin-resistant Staphylococcus aureus at a Saudi university hospital. Microb Drug Resist; 7: 413–416.2001. |
| [20] | Stenhem M., Ringberg H., Larsson L., Olsson- Liljequist B., Haeggman S., and Ekdahl K. Epidemiology of methicillin-resistant Staphylococcus aureus (MRSA) in Sweden 2000–2003, increasing incidence and regional differences. BMC Infect. Dis. 6:30.2006. |
| [21] | Balkhy HH, Memish ZA, Almuneef MA, Cunningham GC, Francis C, Fong KC, Nazeer ZB, Tannous E. Methicillin-Resistant Staphylococcus aureus: A 5-Year Review of Surveillance Data in a Tertiary Care Hospital in Saudi Arabia. Infect Control Hosp Epidemiol; 28:976-982. 2007. |
| [22] | Al-Tawfiq JA. Incidence and Epidemiology of Methicillin-Resistant Staphylococcus aureus Infection in a Saudi Arabian Hospital, 1999-2003. Infect Control Hosp Epidemiol; 27:1137-1139.2006. |
| [23] | Kaplan SL,Versalovic J, Gonzalez BE,. Three-year surveillance of community-acquired Staphylococcus aureus infections in children. Clin Infect Dis.; 40(12): 1785-1791. 2005. |
| [24] | Kim HB, Park WB, Lee KD, Choi YJ, Park SW, Oh MD, Kim EC, Choe KW. Nationwide Surveillance for Staphylococcus aureus with Reduced Susceptibility to Vancomycin in Korea. J Clin Microbiol,Vol. 41, No. 6.p. 2279–2281.2003. |
| [25] | Lulitanond A, Chanawong A, Sribenjalux P, Kaewkes W, Vorachit M, Chongtrakool P, Leumsai D, Monpou P. Detection of heterogenous, intermediate vancomycin- resistant Staphylococcus aureus hVISA using low concentration vancomycin disks. Southeast Asian J Trop Med Public Health, Vol. 37 No. 4, 761- 767.2006. |
| [26] | Song J., et al and the Asian Network for Surveillance of Resistant Pathogens (ANSORP) Study Group, Emergence in Asian Countries of Staphylococcus aureus with Reduced Susceptibility to Vancomycin, Antimicrobial agents and chemotherapy, Vol. 48, No. 12 p. 4926–4928.2004. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML