-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Microbiology Research
p-ISSN: 2166-5885 e-ISSN: 2166-5931
2013; 3(1): 25-31
doi:10.5923/j.microbiology.20130301.04
Potassium Solubilization by Rhizosphere Bacteria: Influence of Nutritional and Environmental Conditions
Priyanka Parmar , S. S. Sindhu
Department of Microbiology, CCS Haryana Agricultural University, Hisar, 125004, India
Correspondence to: S. S. Sindhu , Department of Microbiology, CCS Haryana Agricultural University, Hisar, 125004, India.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Potassium (K) is the third major essential macronutrient for plant growth. The concentrations of soluble potassium in the soil are usually very low and more than 90% of potassium in the soil exists in the form of insoluble rocks and silicate minerals. Rhizosphere bacteria have been found to dissolve potassium from insoluble K-bearing minerals. In this study, bacterial isolates were obtained from wheat rhizosphere on modified Aleksandrov medium containing mica powder as potassium source. Twenty bacterial strains, among 137 cultures tested, showed significant potassium solubilization on mica powder supplemented plates and the amount of K released by different strains varied from 15 to 48 mg L-1. In glucose amended medium broth, bacterial strains WPS73 and NNY43 caused 41.0 and 48.0 mg L-1 of K solubilization. Bacterial strain WPS73 caused maximm solubilization (49.0 mg L-1) at 25°C whereas bacterial strain NNY43 caused maximum solubilization at 30°C. K solubilization was found more when bacterial strains were grown in medium broth with pH 7.0. Maximum K solubilization occurred when KCl was used as a potassium source followed by K2SO4. These results suggested that the environmental conditions could be optimized for growth of potassium solubilizing bacteria and these bacterial cultures could be exploited for plant growth improvement under field conditions.
Keywords: Rhizosphere Bacteria, Potassium Solubilization, Mica Powder, Sugars, Environmental Conditions
Cite this paper: Priyanka Parmar , S. S. Sindhu , Potassium Solubilization by Rhizosphere Bacteria: Influence of Nutritional and Environmental Conditions, Journal of Microbiology Research, Vol. 3 No. 1, 2013, pp. 25-31. doi: 10.5923/j.microbiology.20130301.04.
Article Outline
1. Introduction
- Potassium (K) is an essential macronutrient and most abundantly absorbed cation that plays an important role in the growth, metabolism and development of plants. Without adequate potassium, the plants will have poorly developed roots, grow slowly, produce small seeds and have lower yields. Although, potassium constitutes about 2.5 per cent of the lithosphere but actual soil concentrations of this nutrient vary widely ranging from 0.04 to 3.0 per cent[1]. Plants absorb potassium only from the soil and its availability in soil is dependent upon the K dynamics as well as on total K content. Out of the three forms of potassium found in the soil, soil minerals make up more than 90 to 98 per cent of soil potassium[2] and most of it is unavailable for plant uptake. The second non-exchangeable form of potassium makes up approximately 1 to 10 per cent of soil potassium and consists predominantly of interlayer K of non-expanded clay minerals such as illite and lattice K in K-feldspars, which contribute significantly to the plant uptake[3, 4]. Release of non-exchangeable K to the third exchangeable form occurs when level of exchangeable and solution K is decreased by crop removal, runoff, erosion and/or leaching[2, 5]. With the introduction of high yielding crop varieties/hybrids and the progressive intensification of agriculture, the soils are getting depleted in potassium reserve at a faster rate. Moreover, due to imbalanced fertilizer application, potassium deficiency is becoming one of the major constraints in crop production. This emphasized the search to find an alternative indigenous source of K for plant uptake and to maintain K status in soils for sustaining crop production[6, 7]. Soil microbes have been reported to play a key role in the natural K cycle and therefore, potassium solubilizing microorganisms present in the soil could provide an alternative technology to make potassium available for uptake by plants[8, 9]. Thus, identification of microbial strains capable of solubilizing potassium minerals quickly can conserve our existing resources and avoid environmental pollution hazards caused by heavy application of chemical fertilizers. A wide range of bacteria namely Pseudomonas, Burkholderia, Acidothiobacillus ferrooxidans, Bacillus mucilaginosus, Bacillus edaphicus, B. circulans and Paenibacillus sp. has been reported to release potassium in accessible form from potassium-bearing minerals in soils[10-13]. These potassium solubilizing bacteria (KSB) were found to dissolve potassium, silicon and aluminium from insoluble K-bearing minerals such as micas, illite and orthoclases, by excreting organic acids which either directly dissolved rock K or chelated silicon ions to bring K into the solution[14-16]. Inoculation with potassium solubilizing bacteria have been reported to exert beneficial effects on growth of cotton and rape[10], pepper and cucumber[17], sorghum[18], wheat[19] and Sudan grass[20, 21]. Similarly, inoculation of maize and wheat plants with Bacillus mucilaginosus, Azotobacter chroococcum and Rhizobium resulted in significant higher mobilization of potassium from waste mica, which in turn acted as a source of potassium for plant growth[22]. Therefore, potassium solubilizing bacteria are extensively used as biofertilizers in Korea and China as significant areas of cultivated soils in these countries are deficient in soil-available K[23]. Thus, application of K solubilizing bacteria as biofertilizer for agriculture improvement can reduce the use of agrochemicals and support ecofriendly crop production [24-27]. Currently, little information is available on potassium solubilization by bacteria, their mechanisms of solubilization and effect of KSB inoculation on nutrient availability in soils and growth of different crops. Sheng and Huang[5] found that potassium release from the minerals was affected by pH, oxygen and the bacterial strains used. The efficiency of potassium solubilization by different bacteria was found to vary with the nature of potassium bearing minerals and aerobic conditions. The extent of potassium solubilization by B. edaphicus in the liquid media was more and better growth was observed on illite than feldspar[19]. Therefore, there are immense possibilities for further increasing the production of crops by application of K-bearing rock materials and potassium solubilizing bacteria as biofertilizers.
2. Materials and Methods
2.1. Isolation of Bacterial Cultures from the Rhizosphere Soil
- Seventy rhizobacterial isolates were obtained from the rhizosphere soil of wheat by serial dilution plate method using modified Aleksandrov medium containing (5.0 g Glucose, 0.5 g MgSO4.7H2O, 0.1g CaCO3, 0.006 g FeCl3, 2.0 g Ca3PO4, 3.0 g insoluble mica powder as potassium source and 20.0 g agar) in 1 litre of deionized water[28]. Soil samples were collected randomly from the rhizosphere of wheat at 60 and 75 days of plant growth from 5 different locations of CCS Haryana Agricultural University, Hisar farm. From each location, samples were collected from six different sites. Five samples were collected from each site (one acre) and pooled together to make the composite sample. The serial dilutions of the soil samples were made up to 10-5 and 0.1 ml of diluted soil suspension was plated on Aleksandrov medium plates. The plates were incubated at 28±2°C in biological oxygen demand (BOD) incubator for 3-4 days. Morphologically different colonies belonging to Pseudomonas and Bacillus were selected[29, 30]. Sixty seven reference bacterial strains were procured from the Department of Microbiology, CCS Haryana Agricultural University, Hisar. The rhizobacterial strains/isolates were maintained by periodic transfer on Aleksandrov agar medium slants. These bacterial cultures were stored at 4℃ in refrigerator for further use.
2.2. Screening of Rhizobacterial Isolates for Formation of K Solubilization Zone
- Potassium solubilization by rhizobacterial isolates was studied on modified Aleksandrov medium plates by the spot test method[31]. Plates of modified Aleksandrov medium (A) having mica powder (insoluble form of potassium) and medium (B) having soluble form of potassium i.e., K2HPO4 were prepared. A loopful of 48-hour old growth of the rhizobacterial strain (10 μL of 106 CFU mL-1) was spotted on above prepared plates. Ten bacterial cultures were spotted on each plate and cultures were spotted in same sequence on both types of medium plates. Plates were incubated at 28±2℃ for 3 days. Detection of potassium solubilization by different rhizobacterial isolates was based upon the ability of solubilization zone formation.
2.3. Quantitative Estimation of Potassium Release
- A loopful of 48 hour old grown bacterial culture was inoculated into 25 ml Aleksandrov medium broth in 50 ml capacity flask containing either of different sugars; glucose, galactose, xylose or arabinose. All the inoculated flasks were incubated at 28±2°C for 10 days. The growth suspension was centrifuged at 7,000 g for 10 minutes in the Hettick Mikro Rapid centrifuge (Tuttlingen) to separate the supernatant from the cell growth and insoluble potassium. One ml of the supernatant was taken in a 50 ml volumetric flask and the volume was made to 50 ml with distilled water and mixed thoroughly. The solution was fed to atomic absorption spectrometer to determine K content[32]. Standard curve was prepared using various concentrations of 10 ppm KCl solution i.e., 0.5, 1.0 and 1.5 ppm. The amount of potassium solubilized by the bacterial isolates was calculated from the standard curve.
2.4. Optimization of Growth Conditions for Efficient K Solubilization
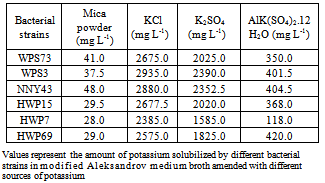
- Solubilization of potassium from mica powder was determined in Aleksandrov medium broth at neutral pH and 28±2℃ temperature. The amendments of different sugars and variation in temperature as well as pH were made to find out optimum conditions for efficient solubilization. Six best efficient potassium solubilizing bacterial strains were used for K solubilization studies. For measuring the effect of carbon sources, rhizobacterial isolate/strain was inoculated into 25 ml of modified Aleksandrov medium broth[28] in which glucose was replaced with either of three different sugars i.e., galactose, xylose and arabinose, respectively. All the inoculated flasks were incubated at 28±2℃ for 10 days. The amount of K released in broths was estimated after incubation in comparison with a set of uninoculated controls. To determine the effect of incubation temperature, Aleksandrov medium broths were inoculated with six selected potassium solubilizing bacterial strains. Cultures were incubated at different temperatures i.e., 25, 35 and 45℃ along with 30℃ for 10 days. To study the effect of pH on K solubilization, the Aleksandrov medium broths were prepared in different pH range i.e., 6.5, 7.5 and 8.5 using N/10 HCl or N/10 NaOH and the broth was buffered with phosphate buffer. After inoculation of bacterial strains, medium broths were incubated at 28±2℃ for 10 days. To understand the effect of potassium release from the minerals, Aleksandrov medium broths were prepared using different forms of potassium i.e., KCl, K2SO4 or AlK(SO4)2.12H2O (3.0 g as potassium source) along with mica powder. Six selected KSB strains were inoculated and inoculated broths were incubated at 28±2℃ for 10 days. After 10 days of incubation, released K was determined in a similar manner as in case of sugars.
3. Results
- In this study, potassium solubilizing bacteria were isolated from rhizosphere soil of wheat plants grown in CCS Haryana Agricultural University farm, Hisar. Bacterial isolates were examined for their ability to solubilize insoluble potassic mineral. The efficient K solubilizers were further subjected to varying carbon sources and incubation conditions to understand the release of K from potassic minerals.
3.1. Isolation of Rhizobacteria from the Rhizosphere Soil
- Seventy bacterial isolates were obtained from soil samples collected from rhizosphere of wheat, grown in field area of the Department of Plant Breeding, Agronomy and Seed Science and Technology in the farm of CCS Haryana Agricultural University, Hisar at 60 and 75 days of plant growth. Serial dilutions of rhizosphere soil samples were made up to 10-5 and dilutions were plated on modified Aleksandrov medium. Rhizobacterial isolates were selected based on morphological and pigment production characteristics. Sixty five reference strains obtained from Department of Microbiology, CCS H.A.U., Hisar, were also screened for potassium solubilization.
3.2. Screening of Bacterial Cultures for Potassium Solubilization
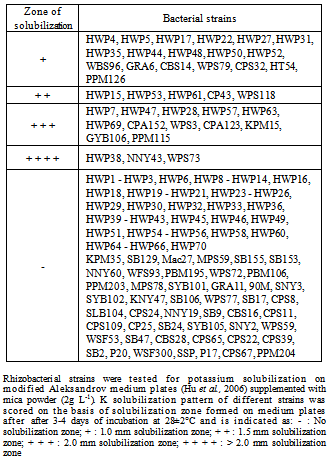
- One hundred and thirty seven bacterial isolates/strains were screened for the potassium solubilization ability using spot test method on modified Aleksandrov medium plates containing either mica powder (A) or KH2PO4 (B). A loopful of 48 hrs old-culture growth of different bacterial isolates was spotted (A) and (B) medium plates and observations were taken after 3 days of growth. It was found that out of 137 rhizobacterial isolates/strains tested, only 20 strains formed significant zone of K solubilization on mica powder containing medium plates (Table 1). Among 20 strains, 15 cultures i.e., HWP7, HWP28, HWP38, HWP47, HWP57, HWP63, HWP69, WPS3, CPA123, KPM15, GYB106, WPS73, NNY43, PPM115 and CPA152 showed large solubilization zone on mica incorporated plates. Five bacterial cultures, namely HWP15, HWP53, HWP61, CP43 and WPS118 showed small solubilization zone (Fig. 1). Three bacterial strains i.e., HWP38, NNY43 and WPS73 showed significant K solubilization zone. Majority of the strains (72.3%) did not cause K solubilization on mica powder containing plates.
|
 | Figure 1. Formation of solubilization zone by bacterial strain HWP47 in modified Aleksandrov medium broth amended with mica powder |
3.3. Optimization of Conditions for Efficient K Solubilization
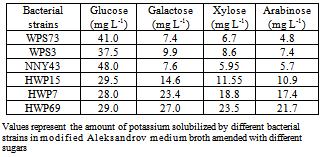
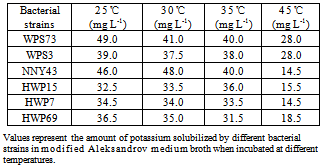
- Six bacterial strains i.e., NNY43, WPS3, WPS73, HWP7, HWP15 and HWP69 were selected based upon the zone size of potassium solubilization. These bacterial strains were further tested for optimization of conditions for efficient K solubilization under varying conditions of carbon sources, temperature, pH and potassium sources used. In glucose amended medium broth, bacterial strains WPS73 and NNY43 caused 41.0 and 48.0 mg L-1 of K solubilization (Table 2). Interestingly, these two bacterial strains also showed large solubilization zone on mica supplemented modified Aleksandrov medium plates (Table 1). When glucose was replaced with other sugars, it was found that K solubilization was comparatively less in galactose, xylose or arabinose amended broth with all the six bacterial strains. Among the three sugars tested, K solubilization by bacterial cultures was more in case of galactose than xylose and arabinose. Maximum K solubilization was observed with strain NNY43 in glucose amended broth whereas bacterial isolates HWP69 and HWP7 showed significant K solubilization with all the four sugars.Different temperatures were used for growth and K solubilization by selected bacterial cultures and it was found that bacterial strain WPS73 caused maximum solubilization (49.0 mg L-1) at 25℃ and K solubilization by this strain decreased at higher temperatures of incubation (Table 3). Bacterial strain NNY43 caused maximum solubilization at 30°C, whereas other bacterial strains showed significant solubilization in the temperature range of 25℃ to 35℃. K solubilization decreased at higher temperature of incubation i.e., 45℃ with all the bacterial strains.
|
|
|
|
4. Discussion
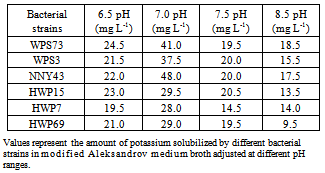
- Nitrogen, phosphorus and potassium are major essential macronutrients for plant growth and development. To enhance crop yields, nitrogenous and phosphatic fertilizers are applied at high rates which cause environmental and economic problems. Therefore, direct application of rock phosphate and rock potassium materials may be agronomically more useful and environmentally safer than soluble P and K fertilizers[33]. However, potassium nutrients are released slowly from the rock materials and their use as fertilizer often causes insignificant increases in the yield of crops[7]. Therefore, concerted efforts are made to understand the combined effects of rock material addition and inoculation of KSB on nutrient availability in soils and growth of different crops. Among the 137 rhizobacterial isolates/strains tested in this study, only 27.7% strains showed K solubilization on modified Aleksandrov medium plates supplemented with mica powder (Table 1). Three bacterial cultures i.e., HWP38, NNY43 and WPS73 formed large zone of K solubilization. Similarly, potassium solubilizing bacteria have been isolated from the roots of cereal crops by use of specific potassium bearing minerals[34] and soil[28, 35, 36]. Alkasandrov et al.[14] isolated different bacterial species which dissolved potassium, silica and aluminium from insoluble minerals. Among the K bearing silicate minerals, mica was found to weather readily[37]. Liu[38] isolated silicate dissolving bacteria B. mucilaginosus CS1 and CS2 from soil and these bacteria also exhibited inhibitory activity on the growth of Gram negative bacteria E. coli. Murali et al.[39] isolated silicate solubilizers using modified Bunt and Rovira medium from soil samples collected from coconut palms. Majority of the silicate solubilizers were identified as Bacillus sp. and Pseudomonas sp. Hu et al.[28] isolated two phosphate- and potassium-solubilizing Paenibacillus mucilaginosus strains KNP413 and KNP414 from the soil of Tianmu Mountain, Zhejiang Province (China). Both the isolates effectively dissolved mineral phosphate and potassium, while strain KNP414 showed higher dissolution capacity even than Bacillus mucilaginosus AS1.153, the inoculant of potassium fertilizer widely used in China. Sugumaran and Janarthanam[40] isolated K solubilizing bacteria from soil, rocks and minerals samples viz., microcline orthoclase, muscovite mica. Among the isolates, B. mucilaginosus MCRCp1 solubilized more potassium by producing slime in muscovite mica. Optimization of conditions for efficient K solubilization by six bacterial strains showed that bacterial strains WPS73 and NNY43 caused 41.0 and 48.0 mg L-1 of K solubilization in glucose amended medium broth (Table 2). K solubilization was comparatively less in galactose, xylose or arabinose amended broth with all the six bacterial strains. Bacterial strain WPS73 caused maximum K solubilization at 25℃, whereas strain NNY43 caused maximum K solubilization at 30℃ (Table 3). K solubilization decreased at 45℃ with all the bacterial strains. K solubilization was found maximum when bacterial strains were grown in a medium with pH 7.0 (Table 4). With increase in pH of the medium, K solubilization decreased. Similar variation in K solubilization ability has been reported by other workers. Sheng and Huang[5] found that potassium release from minerals was affected by pH, dissolved oxygen and bacterial strain used. The content of potassium in solution was increased by 84.8 to 127.9 per cent by inoculation of bacteria as compared with the control. Potassium solubilizing bacterial strains resulted in release of 35.2 mg L-1 potassium in 7 days at 28°C at pH range from 6.5-8.0. Badr[41] studied potassium and phosphorus solubilization capacity of silicate solubilizing bacteria and it ranged from 490 mg to 758 mg L-1 at pH 6.5 to 8.0. Sugumaran and Janarthanam[40] showed that K solubilizing activity of the five slime producing bacterial isolates varied from 1.90 mg to 2.26 mg L-1 from acid leached soil. MCRCp1 was found to have maximum activity (2.26 mg L-1) to dissolve the silicate than other isolates. With the replacement of mica powder in the medium with other potassium sources, maximum K solubilization was observed in KCl and K2SO4 amended medium broth than AlK(SO4)2.12H2O and mica powder containing samples (Table 5). Similarly, the efficiency of potassium solubilization by different bacteria was found to vary with the structure and chemical composition of the potassium bearing minerals[42, 43]. The extent of potassium solubilization by B. edaphicus in the liquid media was more and better growth was observed on illite than feldspar[19]. These results suggested that effective K-solubilizing and plant-growth promoting bacterial-plant systems must be tested further in controlled vegetation experimental designs with specific consideration of soil type, plant types grown and the environmental factors[10, 44]. Moreover, competitive and effective bacterial strains must be selected from the pool of indigenous soil bacteria which could be adopted to the particular conditions of the inoculation site[45]. Thus, potassium solubilizing bacteria isolated in this study could be tested for use in the amelioration of K-deficient soils and may lead to an alternative source for improvement of K nutrition in sustainable agriculture.
5. Conclusions
- Potassium availability to crop plants in soil is generally low since nearly 90 to 98 per cent of total potassium in the soil is in unavailable mineral forms. Moreover, fixation of added nutrients/fertilizers in soil reduces the efficiency of applied P and K fertilizers and thus, a large quantity of added fertilizers become unavailable to plants. Rhizosphere microorganisms contribute significantly in solubilization of bound form of soil minerals in the soil[6, 11, 46]. In this study, different K solubilizing bacteria were isolated from rhizosphere soil samples collected from wheat. Among 137 isolates/strains tested, 20 strains were found to solubilize potassium from mica on Aleksandrov medium supplemented with mica. The amount of K released by the isolates/strains ranged from 15 mg to 48 mg L-1. Six efficient rhizobacterial strains were further examined for optimization of conditions for K release. It was found that maximum solubilization occurred with glucose as carbon source and at 25℃ temperature of incubation, and at medium pH 7.0. Potassium solubilization was found maximum when KCl was used as potassium source followed by K2SO4 and less solubilization was found in mica powder. These results suggested that efficient potassium solubilizing bacterial strains could be further exploited for plant growth improvement under field conditions.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML