-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Microbiology Research
p-ISSN: 2166-5885 e-ISSN: 2166-5931
2013; 3(1): 19-24
doi:10.5923/j.microbiology.20130301.03
In vitro Antimicrobial Activities of Endophytic Fungi Isolates from Medicinal Tree - Melia azedarach L.
Kaushal Kanwer Shekhawat , D.V. Rao and Amla Batra
Plant Biotechnology Laboratory, Department of Botany, University of Rajasthan, Rajasthan, Jaipur, 302004, India
Correspondence to: Kaushal Kanwer Shekhawat , Plant Biotechnology Laboratory, Department of Botany, University of Rajasthan, Rajasthan, Jaipur, 302004, India.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
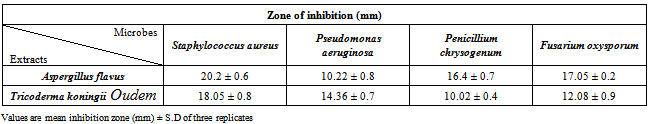
Endophytes, found ubiquitous in all plant species in the world, contribute to their host plants by producing plethora of substances that provide protection and ultimately survival value to the plant. Many researchers have proven that endophyte is a new and potential source of novel natural products for exploitation in modern medicine, agriculture and industry. So far, a great number of novel natural products possessing antimicrobial activities have been isolated from endophytes. These are endophytic microorganisms that inhabit the intercellular spaces of plant tissues and are often responsible for antimicrobial production. The aim of the present study was to screen for antimicrobial activity in two endophytic filamentous fungus Aspergillus flaves and Trichoderma koningii Oudem isolated from surface sterilized leaves and root of Melia azedarach L. and exhibited significant inhibitory activity on the test microorganism by using agar diffusion method. The strongest antimicrobial activity was exhibited by Aspergillus flavus against Staphylococcus aureus where as also in Trichoderma koningii Oudem, with 20.2 ± 0.6 and 18.05 ± 0.8 inhibition, respectively. The two endophytic fungi isolated from leaves and roots tissues of Melia azedarach L. as well as their antimicrobial activity detected in the crude fungal extracts cultures were being reported for the first time.
Keywords: Endophytic Fungi, Melia Azedarach L. Antimicrobial Activity
Cite this paper: Kaushal Kanwer Shekhawat , D.V. Rao and Amla Batra , In vitro Antimicrobial Activities of Endophytic Fungi Isolates from Medicinal Tree - Melia azedarach L., Journal of Microbiology Research, Vol. 3 No. 1, 2013, pp. 19-24. doi: 10.5923/j.microbiology.20130301.03.
Article Outline
1. Introduction
- Microbial endophyte is a microbial colony which resident inside of healthy plant tissues. Endophytic microbes have been recognizing as a chemical producer with a broad range biological activity.World health problems caused by drug-resistant bacteria and fungi are increasing. An intensive search for newer and effective antimicrobial agents is needed. Endophytic fungi have been recognized as useful sources of bioactive secondary metabolites[1],[2]. A recent complete study has indicated that 51% of biologically active substances isolated from endophytic fungi were previously unknown[1]. Many endophytic fungi have the ability to produce antimicrobial substances[4-11]. The mis-usage of antibiotics since the “Golden Age of Antibiotics” in 1950s had caused the threats of antibiotic resistant “super bugs”. Hence, today, many academic institutions are investing necessary resources to search for new natural products with significant pharmacological activity. Majority of the organisms containing new metabolites are disappearing rapidly and screening of the natural products has become costly and laborious process. Endophytes, which occupy a unique biotope with global estimation up to one million species, are a great choice to avoid replication in the study of natural products[3]. Melia azedarach L. (Meliaceae) commonly known as Mahanimba, Ramyaka, Dreka, Karmuka, Keshamushti, Persian lilac and White cedar. M. azedarach L. is a native of West Asia and now naturalized throughout the warm countries. In India, it grows wild in the Sub - Himalayan tract up to 1800 m. The various parts of the tree are reputed to have the same therapeutic values as those of neem tree (Vaidyaratnam PSV, 1994). However, increasing use of Melia azedarach L. in recent years for making traditional medicine. Since the endophytes can be found in nearly all living plant species, a scientific basis in plant selection is necessary for the study of endophytes, to isolate microorganism with pharmaceutical potential. Previous history of the plant’s extensive use in traditional medicines can be criteria for plant selection[3]. As part of our continuing efforts towards finding new antimicrobial compounds, Melia azedarach L., commonly known as Bakhan in India, was used in this study. The photochemistry and pharmacological studies conducted on this medicinal herb have successfully isolated several natural products including diterpenes, monoterenes, triterpenes, saponins, flavonoids hexoses, organic acids, rosmarinic acid, chromene and myoinositol[12][13]. However, therapeutic effect of this medicinal herb is due to the polyphenols. Numerous reports are available on the pharmaceutical potentials of Melia azedarach L. wide range of biological activities including diuretic activity, antihypertensive, anti-inflammatory, antibacterial, antifungal,hepto-protective, antioxidant, antitumor, anti-allergic, and antimalarial activity[12][14].However the medicinal values of their endophytes have not been investigated. The objective of this study was to isolate endophytic fungi from Melia azadarch L. and to study the antimicrobial activity of the isolates on various pathogenic microorganisms.
2. Method and Materials
2.1. Plant Sample Collection
- Mature healthy plant leaves and roots were collected by sampling from different parts of the tree growing in the University Nursery at Rajasthan University, Jaipur. All samples were stored in sterile polythene bags and samples were used to isolate endophytic fungi within 12 hours of collection.
2.2. Isolation of Endophytic Fungi
2.2.1. From Leaves
- Discs of leaves (0.5 cm diameter) were cut using a sterile scalpel. Samples were washed thoroughly in running tap water. Surface of the plant tissues was treated by following the methodology of Murali et al.[15]. Plant tissues were immersed in 75% ethanol for 1 min and in an aqueous solution of sodium hypochlorite (2.5% available chlorine) for 15 min, followed by washing with 70% ethanol for 5 sec. The tissues were then rinsed in sterile distilled water and allowed to surface-dry under sterile conditions. The surface-sterilized samples were placed on Petri dishes containing Potato Dextrose Agar (PDA) supplemented with streptomycin (100 μg/ml) to inhibit bacterial growth and incubated at room temperature at around 25℃.
2.2.2. From Roots
- Be present concerned to isolate endophytic fungi only from within roots that appeared healthy according to the definition of Hooker et al.[16]. They observed discoloration and darkening of roots as they approached mortality. Roots were surface sterilized to kill mycelium and spores on the root surface without eliminating mycelium growing in the outer cortex. Paler roots of healthy appearance were washed in tap water toughly remove soil particle from the root tissue than cut away as close to the shoot base as possible, and surface sterilized in a 72% ethanol solution for 30 s, transferred to a solution of 10% sodium hypochlorite for 10–15 min, and rinsed four in sterile distilled water for four and five times. The roots were then dried in a stream of sterile air (to reduce bacterial growth). The sterilized fine roots were cut into 0.3 to 0.5 cm segments, plated on modified medium PDA containing 1 ml 10% chloramphenicol in ethanol, 400 ml 0.5% dichloran, 10 g sucrose, 10 ml 1% Triton X-100 and 990 ml sterile distilled water. Dichlorans (a dye)[17] and Triton (a nonionic detergent)[18]. The processes reduce the radial growth rate of fungal mycelia and prevent rapidly growing species from over-growing and obscuring the slower growing species. Eight root segments were placed on each plate, and for each quadrate a total of 15 plates were inoculated. Plates were incubated in diffuse daylight at room temperature (20–25℃). Root pieces exhibiting fast growing rapidly sporulating fungi were removed, while slower growing fungi select and were sub cultured. After 3 weeks the number of colonies per plate was recorded, colonies re-isolated where necessary, and each identified to species level where possible.
2.3. Identification of Isolated Endophytic Fungi
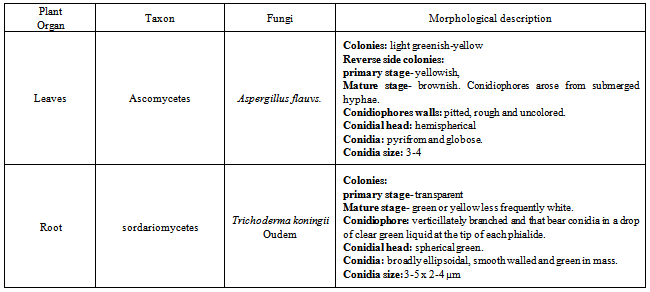
- Cultural and morphological characters of the fungus for studying the cultural and morphological characters, the fungus was grown on PDA. Cultural characters such as color and nature of the growth of the colony were determined by visual observation. Morphological characteristics of the fungus like mycelia, conidiophores and conidia were microscopically studied under the light microscope. Mycelia, conidiophores and conidia produced by the fungus in the culture were examined under the microscope.For the present investigation only two endophytic fungi (Trichoderma koningii Oudem and Aspergillus flavus) were used study of antimicrobial activity among all identified fungi.
2.4. Fermentation and Extraction
- Each of the endophytic fungi isolated from different plant part as leaves and root of Melia azedarach L. In the first stage, these fungi were grown in submerged culture and in the second stage they were grown as stationary culture. These fungi were grown on PDA at 25℃ for 7 days. Seven day-old cultures on PDA of the endophytic fungi were inoculated into Malt Extract Both (MEB) in Erlenmeyer flasks 250 ml, followed by stationary condition and incubated for 30 days at 25℃. After 3 weeks of incubation the culture was harvested and passed through four layers of muslin cloth to separate the mycelia mat from the culture filtrate. The fermentation broth of each fungal endophyte was filtered, and the filtrate was extracted three times with methanol at room temperature. Evaporation of the extracted solution was done in a rotary evaporator. The broth culture was filtered to separate the filtrate and mycelia. The filtrate was used for preliminary testing of antimicrobial activity.
2.5. Microorganisms Used
- Clinical laboratory bacterial isolates of Staphylococcus aureus (Gram +ve) and Pseudomonas aeruginosa (Gram -ve) and fungal isolates viz. Penicillium chrysogenum and Fusarium oxysporum were collected from the stock cultures of Microbiology Laboratory, SMS Medical College, Jaipur India.
2.6. Antimicrobial Assay
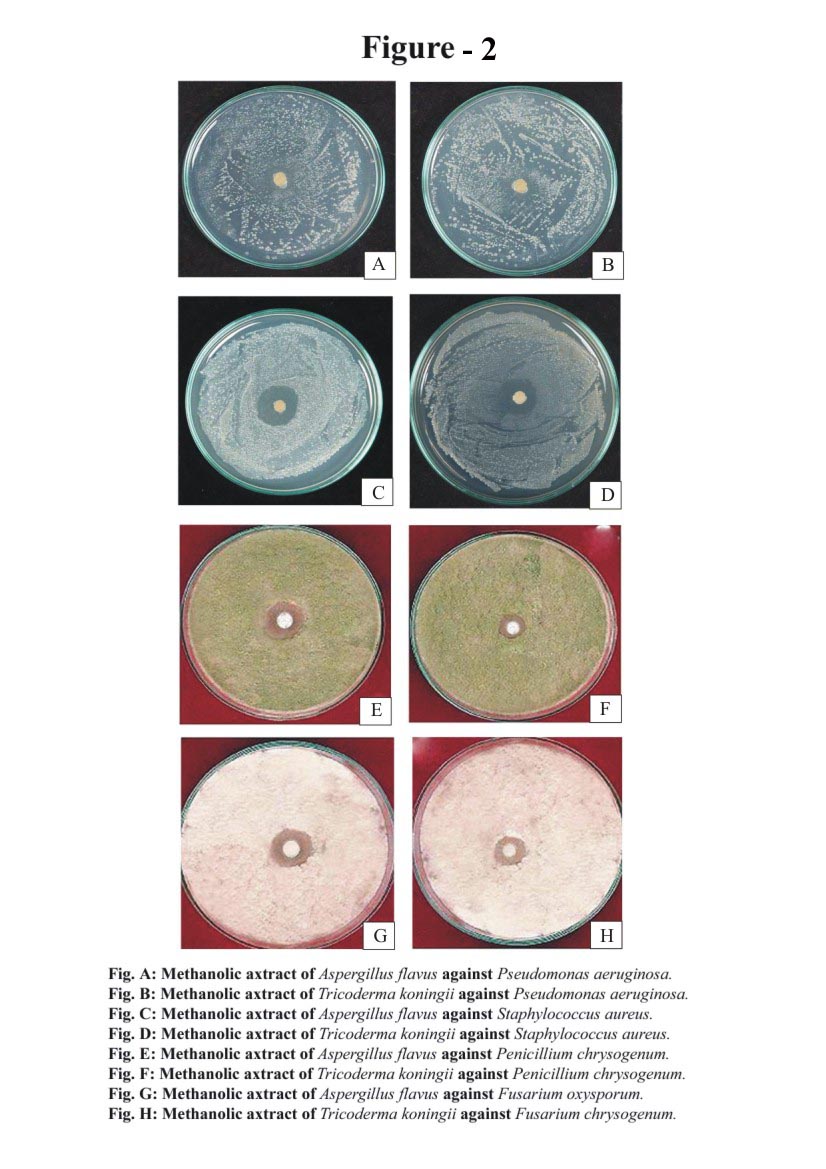
- The antibacterial screening of methanolic extracts of fungi were carried out by determining the zone of inhibition using agar diffusion method[19][20]. The strains of microorganisms obtained were inoculated in conical flask containing 100 ml of nutrient broth. These conical flasks were incubated at 37℃ for 24 hours. The extracts were dissolved in methanol, which was already tested for antibacterial activity against all test microorganisms and found to have no antimicrobial activity. The extracts were made as 50 mg/ml concentration solutions and finally sterilized by filtration using 0.45 µm Millipore filters. The sterile discs (6 mm in diameter) were impregnated with 2.5 µl of above extract solution and placed in inoculated agar. These plates were firstly placed at low temperature (approximately 28℃) for one hour and consent to the maximum diffusion of compounds from the test discs to the agar plates and later incubated at 37℃ for 24 hour in case of bacteria and 48 hours for fungi, after which the zone of inhibition could be easily observed. Three replicates of each test extracts were examined and the mean values were then recorded. The inhibition zones produced by the endophytic fungi extracts.
3. Results and Discussion
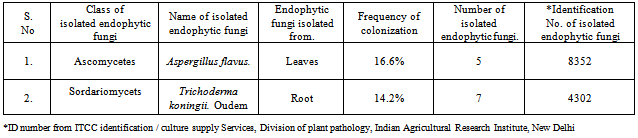
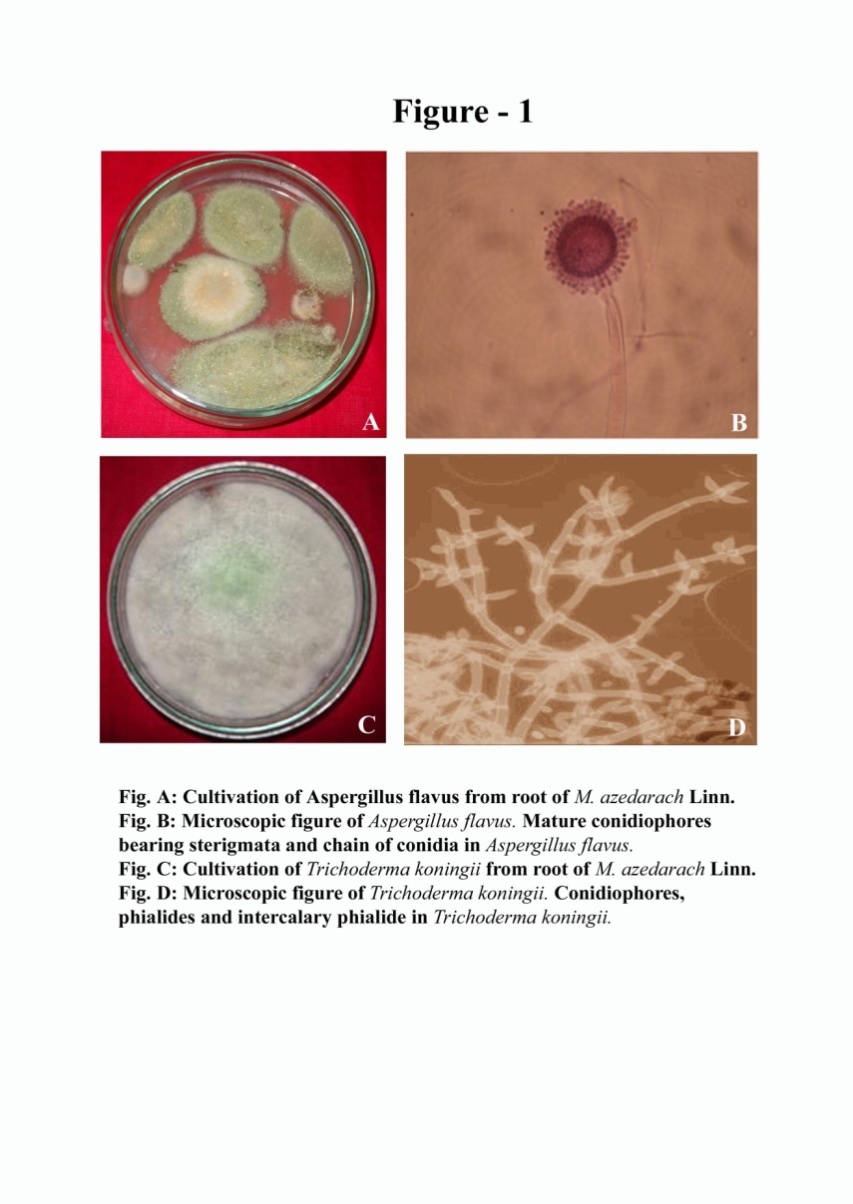
- Total 35 endophytic fungi isolates and identified from each of the leaves and root surface sterilized tissue of Melia azedarach L. (Table-1) In this process total 970 leaves and root samples (leaves and root segments) were use. In which one species of Aspergillus flavus (Figure 1; A) and one species of Trichoderma koningii Oudem (Figure 1; C) were belonging to class Ascomycetes and sorderiomycetes, respectively. Preliminary results on Melia azedarach L. leaves and root tissue isolation show only 2 classes of fungi which produced sterile mycelium (Figure 1; B-D) (Table -2). The fungal colony is identified on the based colony morphology and examination of spores and fruiting bodies. The identification under light microscopes showed presence Aspergillus flavus and Trichoderma koningii Oudem are the dominant mycoflora from the leaves and roots of Melia azedarach L. These both fungi were used for their antimicrobial activity on some pathogenic microbes (S. aureus, P. aeruginosa, P. chrysogenum and F. oxysporum) by agar diffusion method. This is the first report on the endophytic fungi (Aspergillus flavus and Trichoderma koningii Oudem) associated with Melia azedarach and their crude fungal extract shows antimicrobial activity.
|
|
|
4. Conclusions
- In conclusion, results from the current studies indicate that endophytic fungi demonstrated a broad spectrum of activities against bacteria (Gram+ve and Gram-ve) and fungi and support its use as a potent antibacterial and antifungal agent.
ACKNOWLEDGEMENTS
- Authors are thankful to UGC for provide financial assistance in the form of a sanction the major research project 34-169/2008 SR to Prof. Amla Batra, Department of Botany, University of Rajasthan, Jaipur. Author are thankful to ITCC identification / culture supply Services, Division of plant pathology, Indian Agricultural Research Institute, New Delhi for the identification of endophytic fungal Species. Special thanks to Roop Narayan Verma for helping me in making photo plate and manuscript.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML