-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Microbiology Research
p-ISSN: 2166-5885 e-ISSN: 2166-5931
2013; 3(1): 1-10
doi:10.5923/j.microbiology.20130301.01
Microbial Spectrum of Fruit in Gondar town Markets, North Western Ethiopia
Asmaru Gultie , Samuel Sahile
Fuculity of Natural and Computational Science, University of Gondar, P.O.Box 196, Gondar, Ethiopia
Correspondence to: Samuel Sahile , Fuculity of Natural and Computational Science, University of Gondar, P.O.Box 196, Gondar, Ethiopia.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Fresh fruits promote good health. They are highly perishable and affected by different microbial contaminates from production up to consumption. The objective of this study was to evaluate the important microbial spectrum of selected fruits and their management from Gondar town market. A total of 64 fruit samples were analyzed and pathogens were detection from the wash and the peel of fruits. The mean aerobic mesophillic count (AMC) ranged from 4.7x108 to 2.5x1010 from the wash and from 2.96x105 to 3.2x109 from the peel. The total coliform count (TCC) ranged from 6.64x103 to 4.89x10 4 from the wash and 3.12x103 to 5.95x103 from the peel. Mold and yeast count ranged from1.63x103 to 3.46x104 from the wash and 8.03x102 to 1.32x103 from the peel. There was statistical significance difference regarding to AMC, TCC and mold and yeast count between the wash and the peel. From 64 samples E. coli was found in 31(48.4%) of fruits, Pseudomonas aeroginosa was isolated from 13 (20.3 %) samples, Salmonella was detected from 25(39.1%) samples, Staphylococcus aureus was identified from 35 (54.68%) samples, Shigella was found in 10 (15.6 %) samples. Majority of pathogens were sensitive to gentamicin and tetracycline. Market, conditions that favor contamination from poor hygiene of the venders, using microbial unsafe container poor handling practice and poor environmental conditions such as sanitarily unsafe marketing environment were identified.
Keywords: E. Coli, Fresh Fruits, Fruit Handlers, Hygienic Condition, Total Coliform, Contamination
Cite this paper: Asmaru Gultie , Samuel Sahile , Microbial Spectrum of Fruit in Gondar town Markets, North Western Ethiopia, Journal of Microbiology Research, Vol. 3 No. 1, 2013, pp. 1-10. doi: 10.5923/j.microbiology.20130301.01.
Article Outline
1. Introduction
- The role of fresh fruits in nutrition and healthy diet is well recognized and in recent years many countries have undertaken various initiatives to encourage consumers to eat more of these products. Fruits supply much needed vitamins and minerals. They play an important role in health through the prevention of different diseases. Consumption of fruit can help to achieve or maintain a healthy body[1]. The health aspect with increasing consumer demands for variety and availability and the change structure of global trade has led to an increase in fruit trade internationally. For many countries fruit products have become valuable, making a substantial contribution to the economy as well as to the health of country population[2].Pineapples, passion fruits, bananas, avocados, citrus fruits mangoes, mandarin, papayas, guava, grapes, asparagus are produced in Ethiopia. Around 47 thousand hectare of land is under fruit in Ethiopia. Banana contributed about 60.6 % of fruit area followed by mango that contributed about 12.61 % of the area[3]. Total fruit production in Ethiopia is about 500 thousand tones. In Ethiopia fruit have significant importance with a potential for domestic and export markets and industrial processing. The main fruits produced and exported are banana, citrus fruits, mango, avocado, papaya and grape fruits[2].Growing and marketing of fresh fruit in Ethiopia are complicated by post harvest losses both in terms of quality and quantity between harvest and consumption. The quality of fresh fruit depends up on the harvesting activities, post harvest handling, transportation and storage[4].Compared with other temperate fruits, tropical and subtropical fruits such as mango, banana, and papaya currently with great problem in storage and transportation because of their perishable nature[5].The production, marketing and consumption of mango, banana, avocado and papaya fruits are restricted due to improper handling, inadequate transport and storage facility, disease problems, and sensitivity to low storage temperature[5].Even though Ethiopia is experiencing huge post harvest losses, very little emphasis has been laid on the postharvest handling[6]. With in all these activities fruits are exposed to microbial contamination through contact with soil, dust, and water. It there for harbor a diverse range of microorganisms[7]. Specific microorganisms affect product safety and quality by increasing fruit loss and might there for pose a health risk for the consumer.Fresh fruits recently have confined as a significant source of plant and human pathogens and chemical contaminants that pose a potential threat to human health worldwide and the contamination is special concern. Because it likely to be consumed raw without processing thus posing a potential food safety any type of microbiologically lethal processing thus passing a potential food safety problems[8]. Poor handling can damage fresh produce, rendering the product susceptible to the growth or survival of spoilage and pathogenic microorganisms. This damage can also occur during harvesting, packaging and transporting. The presence of cut and damaged surfaces provides an opportunity for contamination and growth of microorganisms and enters into plant tissues[9].In Gondar town fruits such as citrus fruits, banana, mango and avocado are common supplementary diets. The fruits are usually available from markets. Especially orange and banana are available in the market throughout the year while the others are often seasonal. The town markets obtain fruits from different governmental and private farm lands that cross many hundreds and thousands kilometers (Personal communication with fruit distributors). In addition, the problem can be enhanced from poor management of fruits in the market. Market, conditions that favor contamination can be raised from poor hygiene of the venders, using microbial unsafe container poor handling practice and poor environmental conditions such as sanitarily unsafe marketing environment.The consequence of the problems is increasing loss of fruit due to microbial spoilage and the existence of some human pathogens[9]. So assessing the microbial spectrum of fruits in the market is important in many aspects.Fruit safety has emerged as an important global issue with international trade and public health implication. In response to the increasing food borne illness government all over the world are intensifying their effort to improve fruit safety[10]. However in Ethiopia no sufficient continuous survey /assessment of fruit safety has been prepared or developed. The researcher has been motivated to fill the gap. Therefore, this study has tried to determine the microbiological quality and management of fruits in Gondar town.The significant of this study is; generate information about the microbial quality of selected fruits in the town for indicate the current health risk; identify basic problems related in management of fruits. In addition, the study provides information for further study.
2. Materials and Methods
2.1. Description of the Study Area
- The study was conducted at University of Gondar. Gondar is located in the north western Ethiopia at a latitude of 120 36’ N, and longitude of 370 28’ E with an elevation of 2080 m. a. s. l. Gondar town is located in the west of Northern Gondar administrative zone which is far from 747 Km from Addis Ababa in the North West direction. Based on the 2005 census the total population of Gondar is 194,773 (97,625 males and 97,148 females). Gondar has mid altitude climate and an average annual max temperature of 27 ℃ and minimum temperature of 16 ℃. This town administration has twenty three urban and 11 rural kebeles (Source: Gondar Statistics office).
2.2. Study Design
- A cross sectional and descriptive survey design were undertaken from the middle of December 2011 to May 2012 to evaluate the microbiological quality and the management of fruits at Gondar town. Within the available resources half of the main fruit shops (16) for microbiological analysis and all the main fruit shops (32) were selected for the interview and observation purpose. Microbiological indicators and pathogens on the wash and from the peel of fruits were analyzed.
2.3. Sampling technique
- In the study area there were four main fruit marketing centers. So representative samples were selected by the combination of simple random sampling and stratifying the town in to four markets cites. The four marketing cites were the major places in which fruit marketing processes were taking place. For each cites 50% of the vendings were taken randomly selected out of the total for the microbiological analysis and a total of sixteen samples were taken per fruit. For the field study random sampling technique was used.
2.4. Sample Collection
- The study was consisting of two parts. The first part was laboratory work which analyzed the microbial indicators and detection of pathogens. The experimental work was conducted in Gondar University Faculty of natural and computational science in the department of biology, applied microbiology laboratory. Four commonly used fresh fruits were taken from four Gondar local markets. The fruits were banana, orange, mango and avocado. A total of 64 fruit samples (16 orange samples, 16 mango samples, 16 avocado samples and 16 banana samples) were purchased from the markets of Gondar town from December 2011 to March 2012. The samples were collected aseptically in a sterilized container.The aerobic mesophilic count, the total coliform count, the yeast and mould count on the surface (wash) and from the peel of fruit were determined using standard plating technique as described by APHA method[12]. The identity of microbes on the surface (wash) and in the peel were identified or characterized by using morphological and biochemical technique. The presences of common pathogens on the surface (wash) and from the peel of fruit were identified using different selective and differential media as described by different standard procedures[13, 14, 15and16].For the field study data was collected using structured interview and observation. For the purpose of this an interview was made with 32 fruit venders and an observation was also made about the sanitary condition of the market or the shop, the hygienic condition of the fruits venders and their handling practices using check list. In addition the handling practices of the people who were engaged for loading and unloading fruits from the vehicles were assessed using observation. The study was conducted from the middle of December 2011 to May 2012.
2.5. Sample Preparation for Microbiological Analysis
- The sample preparation and microbiological analysis was consisted of two parts. The two parts were sample preparation and microbiological analysis at the surface of fruit or the wash and from the fruit peel. The two parts were done side by side.
2.5.1. Sample Preparation for Microbiological Analysis on the Fruit Surface (Wash Part)
- Sample preparation for aerobic plate count, total coliform and fungi count, and pathogen detection 25 g of the sample was aseptically weighted and rinsed thoroughly in 250 ml of distill water for five minutes. The washed fruits were removed aseptically and saved for the microbial analysis of the peel. The wash was analyzed according to standard procedure.Aerobic mesophilic count (AMC)The aerobic mesophilic count of all the fruits at surface was determined by standard plate count method as described by APHA on nutrient agar medium (Don whitely eqp. Pvt.ltd-India). One mL of the sample was serially 10 fold diluted in 0.1 % of buffer peptone water (Don whitely eqp. Pvt.ltd-India). 0.1 mL from each serially diluted sample (10-5 to 10-10) was pour plated on standard plate count agar medium. The samples were incubated at 37 0C for 24 hours. After incubation plates with colonies between 25-250 were counted[12].Enumeration of coliformsTotal coliform count (TCC)Total coliforms were determined using both the most probable number (MPN) method and enumeration using L-EMB agar (Don whitely eqp. Pvt.ltd- India) as recommended by APHA[12]. For the MPN method one mL of the sample was diluted up to a factor of 10-4 were made. Aliquot dilutions were inoculated in to triplicate tubes containing lactose broth (International diagnostics groups PIC, Lancashier, UK ) and were incubated at 35 ℃ for 24 hours. Tubes with gas formations at the end of the incubation periods were planted in to brilliant green bile broth (BGBB) (Oxoid, Unipath Ltd., Basing Stoke, and Hampshire, England) and incubated. Those tubes which formed a gas as a result of incubation process were evaluated according to the MPN table and results of a test were reported as MPN per mL of sample[17].For the enumeration of total coliform using Eosin Methyl Blue agar 0.1 mL from each serially diluted sample (10-1 to 10-5) was pour plated on Eosin Methyl Blue agar (L-EMB) (Himedia,M022S India). The plates were incubated at 37 ℃ for 24-48 hour. After incubation colonies between 25- 250 were counted. The colonies were transferred into a slant media and characterized by biochemical tests such as indol test, catalase, urease, methyl red test, VP test and TSI test.Isolation and identification of E. coliThe presence of E. coli was also confirmed by using the following standard method. One mL of the sample was diluted in nine mL of lactose broth and incubated at 37 ℃ for 24 hours. The sample was examined for gas formation. After shaking the lactose positive broth one loopful sample from the tube was transferred in to 10 mL of E. coli (EC) (DIFCO 0314-01-0) broth. The sample was incubated for 24 hours at 45 ℃. Culture which gas positive from EC broth one loop of culture was streak on L-EMB agar (Himedia, M022S India). The culture was incubated at 35 ℃ for 18-28 hours. Dark center colonies with metallic sheen were considered as indicative of E .coli[14]. The culture was further confirmed by indol test, MRVP test, TSI test citrate, catalase and urease test. In general For identification procedure (for the tested pathogens) we have used Bergey's manual determinative microbiology as basic procedures.Mold and yeast countPotato Dextrose Agar (PDA) (Don whitely eqp. Pvt.ltd-India) in presence of 10 % tartaric acid was used. One ml of the sample was serially 10 fold diluted in 0.1 % buffer peptone water. 0.1 mL from each dilution (10-1 to 10-4) of the serially diluted sample was plated on PDA in duplicates. The plates were incubated at 21 ℃ for 5-7 days. After incubation colonies between 10 -150 were counted. Then based on their macroscopic structure the colonies were sub cultured and incubated on new PDA agar and slant for further characterization[18].The identification of the isolates fungi was done according to the microscopic methods of[19]. A drops of lacto phenol cotton blue stain (Don whitely eqp. Pvt.ltd-India) was placed on a clean slid and with the aid of mounted needle, a small portion of the mycelium from the fungal cultures was removed and placed in the drop of the stain.The mycelium was spread very well on the slid and a cover slip was gently lowered on it. The slid was examined under the microscope. The observation was done at low and high power objectives of the microscope. Morphological characters of the hyphae, asexual reproductive structures were observed and recorded.Pathogen detectionDifferent selective and differential media were used for the isolation and identification of pathogens on the surface (wash) and from the peel of fruit. The isolation and identification of Pathogen such as Pseudomonas spp, Salmonella spp, Shigella spp , staphylococci spp. and Listeria monocytogen, performed using standard procedures.PseudomonasFor the isolation of Pseudomonas, Pseudomonas agar P base (Don whitely eqp. pvt.ltd.-India) in presence of glycerol was used. One mL of the sample was pour plated on the media and incubated at 30 ℃ for 24-48 hours. The typical colonies which changed the colour of the media to blue green were considered as indicators of Pseudomonas aeuroginosa. The suspected colonies were transferred to nutrient broth for incubation at 37 ℃ for 24 hours. The cultures were further characterized using different biochemical tests such as indole test, citrate test, oxidase test, TSI test and MRVP test[15].Salmonella and ShigellaFor the isolation of salmonella and shigella, salmonella-shigella (SS) agar was used (Don Whitley eqp. pvt.ltd.-India). 0.1 mL of the sample was pour plated on the salmonella-shigella agar and incubated at 37 ℃ for 18-24 hours. Then colourless colonies with and without black center were transferred to nutrient broth for incubation at 37 ℃ for 24 hours. The cultures were characterized by motility test, indol test, MR, VP, TSI test and urease test[16].Staphylococcus aureusStaphylococcus aureus was isolated using manitol salt agar (MSA) (Himedia, M400086, India). 0.1mL of the sample was pour plated on MSA and incubated at 37 ℃ for 24-48 hours. Colonies that changed the medium to yellow and others that did not change the colour of the medium were transferred to nutrient broth and incubated at 37 ℃. The cultures were further confirmed using coagulase test, catalase test, MRVP test, oxidase and indole test[13].Listeria monocytogenFor the identification of Liseria, one ml of the sample was transferred in to 9 ml of Listeria enrichment broth(Himedia, M888, India) and incubated at 37 ℃ for 24-48 hours. A loop full enrichment sample was streaked onto Listeria identification agar base (PALCAM) (Himedia, M1064, India) and incubated at 36 ℃ for 48 hours[20].
2.5.2. Sample Preparation for Microbiological Analysis from the Peel of Fruit
- For the preparation of sample from the fruits peel 25 g of the washed fruit peel from the previous experiments was aseptically weighted and diluted in 225 ml of 0.1 % of buffered peptone water and homogenized in stomacher for 2 minutes at normal speed. Then the aerobic plate count, total coliform count, fungi count, isolation and identification of pathogens were done using the same procedures and experimental conditions of the microbiological analysis from the wash part.
2.6. Antimicrobial Susceptibility Tests
- After the pathogenic bacteria were isolated and identified antimicrobial susceptibility testing was conducted on Pseudomonas aeruginosa, Staphylococcus aureus, Salmonella spp., Shigell spp. and E. coli by agar disc diffusion standard method using Mueller-Hinton agar (Sisco Research Laboratories pvt. Ltd.-India)[21]. The media was prepared according to the instruction of the manufacturer. The isolate colony cultures were transferred to saline broth, vortexed thoroughly and the bacterial suspension was compared to 0.5 McFarland standards. Then the culture was streaked on the medium within 15 minutes and 10 µg penicillin, 30 µg of tetracycline, 10 µg ampicillin and 10 µg gentamycin discs (Oxoid ltd.) were applied on the plate individually. The plates were incubated at 35 0 C for 16 to 18 hours.The diameter of the zone of complete inhibition was measured and recorded in millimeter. Finally zone of inhibition was compared with the zone size interpretative table and recorded as susceptible, intermediate or resistant to each antimicrobial tested[22].
2.7. Data Analysis
- The data collected from all the experiments and field study were subjected to the analysis of appropriate statistical and SPSS-16 computer software. The data collected from the experiments were subjected to the analysis of variance with the appropriate statistical computer software. Average values were used for duplicates the data and all the countable dilution were used to calculate the average number of colonies in terms of colony forming unit per gram (cfu/gm) or cfu/mL for aerobic plate count and mold and yeast count, most probable number per gram (MPN/gm) or MPN/mL for the statistical estimation of coliform and the appropriate statistical interpretation methods were used for the specific type of data obtained from the experiments and the result of field study. P < 0.05 was taken as statistically significant association.
3. Results and Discussion
3.1. Microbiological Analysis of Fruits
3.1.1. Microbiological Analysis on the Wash of Fruits
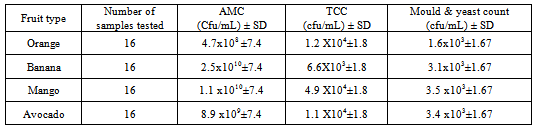
3.1.1.1. Aerobic Mesophilic Count, Total Coliform and Yeast and Mold Count
|
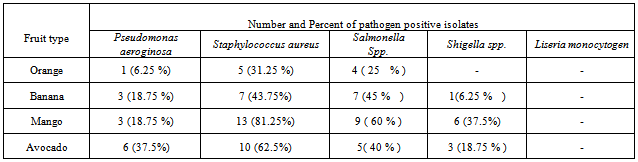
3.1.1.2. Pathogen Detection
- Fruits during growth, harvest, transportation and handling at any stage can be contaminated with pathogens from human and animal sources[26]. So in this study an attempt was also made to assess or identify pathogens using different differential and selective Media. The result as shown in the table 10 most fruit samples were contaminated by more than one pathogen.
|
- Among the Pseudomonas species the pathogenic pseudomonas auroginosa was found in 13 (20 %) of the fruit samples. The highest and lowest prevalence frequency of this pathogen was identified from avocado and orange respectively. Different studies have shown that Pseudomonas species can be found chiefly in soil, water, in the normal flora of people colon and their skin. Because of various sources fruits could be contaminated by this bacterium[15].From all fruit samples 35 (54.7 %) were contaminated by Staphylococcus aureus. This includes 5 orange, 7 bananas, 13 mango and 10 avocado samples were contaminated with Staphylococcus aureus. The presence of Staphylococcus aureus in this study indicated that the poor personal hygienic practice and related factors. This may be because fruit handlers do not use glove, hair nits and different fruit buyers touched by their hands for sorting healthy fruits during marketing.Similar results were found in Nigeria in which from 10 fruit samples 7 fruits were contaminated by Staphylococcus aureus[27]. The presence of S. aureus pathogens on fruits can cause health risk for the consumers.In recent year fresh fruits gained concern as a vehicle of transmission of Salmonella where contamination can occur at multiple steps along the food chain[28]. In this study Salmonella isolate was identified in 25 (39 %) fruit samples. 9 mango samples, 7 banana samples, 4 orange and 5 avocado samples were contaminated by Salmonella. Regardless of the type of fruit from a total of 16 fruits samples 9 samples were Salmonella positive from arada market. However from Piassa only 5 samples were contaminated. Salmonella isolate was not identified from all orange samples from Piassa and kebele 18 markets.From this study Shigella was identified from 10 (15.6 %) samples. 1 banana, 6 mango and 3 avocado samples were contaminated by Shigella. The pathogen was not identified from orange samples (table 2). The maximum and minimum number of contaminated fruit samples were identified from azezo market (5 samples) and kebele 18 (1 samples) respectively. Mango samples were identified as more contaminated than others. The presence of Salmonella and Shigella on raw fruit had been reported by[29]. L. monocytogen was not found in all samples of the study area.
3.1.2. Microbiological Analysis from the Peel
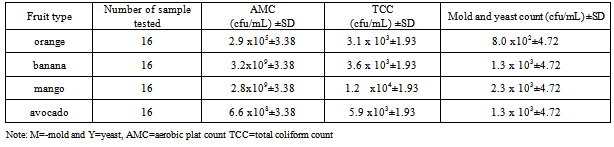
3.1.2.1. Aerobic Mesophilic Count, Total Coliform , Mold and Yeast Count
- Like the microbiological analysis of the wash part the same producers and experimental conditions were performed for the microbiological analysis of the peel. The aerobic mesophilic count (AMC), total coliform count, yeast and mold count of the peel are given on table 3. Like the wash the peel also contained large bacterial load.The mean aerobic mesophilic plate count from the peel ranged from 2.96 x 105 to 3.2 x 109 cfu/g. The highest and the lowest AMC means were found on mango fruit (1.6x1010 cfu/g ) and orange (1.2x105) respectively. From all fruit samples the highest aerobic mesophilic count was identified from mango from Arada market while the least microbial load was from orange at piassa market. The cause for the low bacterial count from orange might be due to low PH of the peel that inhibits the growth of the microorganisms. During the experimental work the PH of the homoginated peel was 3.5-4.1.From the statistical analysis of AMC, the p value for the location of the markets was 0.03 which is less than 0.05. This indicated that the presence of significant difference between the marketing area on the AMC. The post Hoc analysis indicated that there was least significance difference on the AMC. The least significance difference was observed between azezo and other areas. The p=0.017< 0.05, there was a significance difference in AMC between fruit types. From post Hoc analysis the least significance difference was observed between orange and banana (p=0.003), banana with avocado (p=0.019).The ANOVA result about the AMC between the means of the peel and the wash was p=0.00 which is less than 0.05. The result shows that there was a significance difference between the two means. Practically more AMC was found from the wash than the peel. This is an important indicator for the importance of washing fruits before consuming.Both the MPN index and the enumeration of total coliform indicated that coliforms were found in the peel of all fruit samples. The MPN ranged 93 MPN/g (orange) from piassa to 1.1x 103 MPN/g (mango) from azezo market. The mean total coliform count using the EMB agar also ranged from 3.1 x 103 to 1.9 x 104 cfu/g.
|
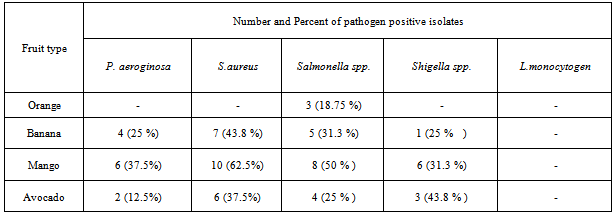
3.1.2.2. Pathogen Detection from the Peel
- The presence and absence of pathogens from the peel was assessed using the same standard experimental procedure which was used for the wash. The result as shown in Table 4 like the wash part different pathogens was identified from the peel. Even though most of pseudomonas species are the microflora of fruits; Pseudomonas auroginosa is a known pathogen for human beings. From this study the pathogenic Pseudomonas auroginosa was found from the peel of 12 fruits. From these fruits 6 were mango,4 banana and 2 were avocado. The pathogen was not identified from the peel of orange. Different studies indicated that Pseudomonas species are found chiefly in soil, water, in the normal flora of people colon and their skin[15].The Staphylococcus aureus was found in twenty three (35.93%) of fruit samples. 7 banana, 10 mango and 6 avocado samples were contaminated by Staphylococcus aureus. The bacteriological study of fresh fruit in Nigeria had shown that among 10 fruit samples 7 fruits were contaminated by Staphylococcus aureus[27]. The presence of this pathogen indicated that the presence of poor handling and hygienic problem of handlers at different stage of fruit handling.In this study Salmonella isolate was identified in 21 (32.8 %) fruit samples. The highest number of contaminated fruits was mango (50 %) and the lowest number was found from orange 3 (18.75%). The bacteria was found from 7 samples at arada, 4 samples at keble 18, 5 samples from azezo and 3 piassa. Salmonella isolate was not identified from all orange samples from pissa and kebele 18 markets.From the study Shigella was identified from 10 (15.62%) samples. This pathogen was found in 1 banana, 6 mango and 3 avocado samples. The pathogen was not identified from orange samples. The maximum and minimum number of contaminated fruit samples were identified from azezo market (8) and kebele -18 (2 samples) respectively. Similar to the wash L. monocytogen was not identified in all fruit samples.
|
|
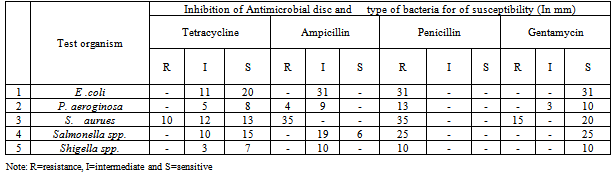
3.1.3. Antimicrobial Susceptibility Test of Isolated Pathogens
- The antimicrobial susceptibility test was conducted on five isolated pathogens by comparing with the control (Table 5). The zone of inhibition diameter for the controls was taken as zero. The zone of inhibition diameter of E. coli was 12-23 mm for tetracycline, less than 10 for penicillin, 14-16 mm for ampicillin and 14-20 mm for gentamicin. Salmonella and Shigella isolates showed 18-21 mm inhibition zone diameter for tetracycline, 5- 8mm for penicillin, 14-16 mm for ampicillin and 15-20 mm for gentamicin.The inhibition zone diameter of P. aeuroginosa was ranged from 10 - 20 mm on tetracycline and gentamicin antimicrobial agents. The pathogen showed 12-14 zones of inhibition for ampicillin and less than 10 mm for penicillin. The inhibition zone diameter of staphylococcus aureus was ranged from 24 to 30 mm for ampicillin, 10-15mm for penicillin and 19-23 mm for tetracycline and gentamicin.The antimicrobial susceptibility test as shown on table 5 indicated that from thirty one isolated E. coli bacteria E. coli 20 (64.5 %) were sensitive to tetracycline while 11 (35.4 %) were showed intermediate. From the examined samples 6 (19.4 %) were sensitive to ampicillin the rest were showed intermediate sensitivity. In this study 25 (80.6 %) of E. coli showed resistance against penicillin. However all the isolated E. coli cultures were sensitive to gentamicin.From the study 7 (53.8 %) isolated P. aeuroginosa isolates were sensitive to tetracycline and 10 (76.9 %) were sensitive to gentamycin. From the isolated P. aeuroginosa all showed resistance to penicillin while 4 were resistance against ampicillin. Studies showed that P. aeuroginosa were susceptible to gentamicin but resistance to ampicillin and penicillin[29].From the samples tested 20 (57.14%) and 13 (37.2%) S. aureus isolate were sensitive to gentamicin and tetracycline respectively. The entire isolated S. aeureus showed resistance to ampicillin and penicillin. The number of Salmonella sample sensitive to tetracycline, ampicillin and gentamycin were 15 (60 %), 6 (24 %) and 25 (100%) respectively. All Salmonella samples were showed resistance against penicillin.All Shigella isolates were sensitive for gentamicin and tetracycline. However all isolated Shigella were resistance to penicillin and all showed intermediate susceptibility to ampicillin.
4. Conclusions
- On the basis of the findings of the study it was concluded that majority of fruit type harbored high microbial load most of which are dangerous to human health such as Pseudomonas aeroginosa, Salmonella spp., E. coli, Shigella spp. and S. aurues. The presence of E. coli in most fruit samples indicated the presence of fecal contamination at any stage of fruit processing. High microbial load was found on the wash and the peel. However there was significant difference for aerobic mesophilic count and total coliform count from the wash and the peel in which more microbial count was found from the wash part. Statistically significance difference in aerobic mesophilic count and total coliform count were identified among the marketing areas which might be due to the presence of different variables that enhanced microbial load.All fruit types in the study area were harbored known post harvest fruit spoilage molds. The presences of such spoilage molds have an economical and health risk in which they are sources of different secondary metabolites. The presence of pathogens in these fruits can cause health risk on the consumers. The identified pathogens were sensitive to tetracycline and gentamicin. However they showed resistance on penicillin and intermediate sensitivity on ampicillin drugs.From the assessment of fruit management a number of deficiencies were identified in the study area. Fruit venders were unaware of the health risk of fruit and causes of fruit contamination. Poor handling practice during storage, measuring or weighting, loading and unloading from the vehicles, unable to use waste collection bin were also identified as deficiencies in most of the study areas. In addition in some fruit marketing areas sanitary deficiency was identified that can increase the probability of fruit contamination.Despite the high microbial counts obtained from the samples in this study it is important to note that the samples did not show any visible sign of spoilage. Thus out ward appearance may not be a good criterion for judging the microbial quality of fruits. So, all fruits should be adequately washed before consumption to reduce the microbial loadThe responsibility to safeguard fruits from contamination is shared by everyone involved from the grower to the consumer. Education and knowledge are tools to improve fruit safety controls. So training the fruit handlers such as fruit venders or sellers and workers engaged for loading and unloading fruits from the vehicles about fruit born diseases, contamination of fruits, handling of fruits and the impact of hygienic problems on the safety of fruits is essential to reduce the contribution of the fruit handlers for contamination in the study area .Supervising and monitoring system on the sanitary condition of fruit marketing areas and the feasibility of transport system should be introduced by concerned body. For the future further studies should be conducted on the areas such as the necessary alternative decontamination methods of fruits and the economical impact of fruit loss.
ACKNOWLEDGEMENTS
- This study was partially funded by University of Gondar, Faculty of natural and computation science, School of Graduate Studies, and Department of Biology. The authors are also thankful to Ato Gashaw , assistant of the applied microbiology laboratory at Gondar University for his dedication and a sense of help to get things done. We also thank Department of biology, microbiology of University of Gondar for allowing us to use the laboratory during the study period.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML