-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Microbiology Research
p-ISSN: 2166-5885 e-ISSN: 2166-5931
2012; 2(6): 176-182
doi: 10.5923/j.microbiology.20120206.04
Cytotoxic Effects and Safety Profiles of Extracts of Active Medicinal Plants from South Africa
Morobe I. C. 1, Mthethwa N. S. 1, Bisi-Johnson M. A. 1, Vasaikar S. D. 1, Obi C. L. 1, 2, Oyedeji A. O. 3, Kambizi L. 4, Eloff J. N. 5, Hattori T. 6
1Department of Microbiology, Walter Sisulu University, 5117 Mthatha, Eastern Cape, South Africa
2Academic and Research Division, Walter Sisulu University, 5117 Mthatha, Eastern Cape, South Africa
3Department of Chemistry and Chemical Technology, Walter Sisulu University, 5117 Mthatha, Eastern Cape, South Africa
4Department of Botany, Walter Sisulu University, 5117 Mthatha, Eastern Cape, South Africa
5Department of Phytomedicine, Faculty of Veterinary Medicine, University of Pretoria, Pretoria, South Africa
6Laboratory Emerging Infectious Diseases, Internal Medicine, Graduate School of Medicine, Tohoku University Sendai, Japan
Correspondence to: Morobe I. C. , Department of Microbiology, Walter Sisulu University, 5117 Mthatha, Eastern Cape, South Africa.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Plant derived antimicrobial compounds that have no or minimal toxicity to host cells are considered candidates for developing new antimicrobial drugs. Safety is therefore critical in the formulation of antimicrobials. The aim of this study was to investigate the cytotoxic effects of some South African medicinal plant extracts. The methanolic and aqueous extracts of nine South African medicinal plants were screened for cytotoxic activities against MAGI CC5+ cells using MTT assay.The nine plant extracts used in the MTT assayrevealed Herb 2 (Cyanthula inculata) as the most potent extract identified with activity of (1.4 Cc50values of 25.6 mg/ml) and induced over 50% of cell deaths, followed by herb 3 (Croton grattismus) and Herb 4 (Cassine trasvaalensis) with activity of (0.2 Cc50 values of3.7 mg/ml) each. The herbs that induced the least cell death, were herbs 5 (Capris tomentosa) and 7 (Hypoxis hemerocallidea), with the activity of (0.05 Cc50 values of 0.9 mg/ml) each. Of the nine plant extracts, Croton grattisimus and Lycium inerme 2(22%), exhibited minimal toxicity on MAGI cells and 7(77.8%) exhibited 50% toxicity. In a similar study 2(22%) of the methanolic extracts exhibited anti-HIV1 IIIB activities and against Mycobacterium tuberculosis (TB) only one medicinal plant extract (Lysium inerme) exhibited 29% activity. In this study, a systematic evaluation of cytotoxic activities of methanolic extracts made from tested medicinal plants showed minimal toxicity on cell lines. Therefore, such plants could serve as sources for natural antimicrobial therapeutic agents.
Keywords: South Africa, Medicinal Plants, Cytotoxic Activity, Antibacterial Activity, Herbs, Antimicrobial Compounds, Respiratory Tract Infections
Cite this paper: Morobe I. C. , Mthethwa N. S. , Bisi-Johnson M. A. , Vasaikar S. D. , Obi C. L. , Oyedeji A. O. , Kambizi L. , Eloff J. N. , Hattori T. , "Cytotoxic Effects and Safety Profiles of Extracts of Active Medicinal Plants from South Africa", Journal of Microbiology Research, Vol. 2 No. 6, 2012, pp. 176-182. doi: 10.5923/j.microbiology.20120206.04.
Article Outline
1. Introduction
- The increasing global prevalence of multi resistant respiratory tract pathogens is becoming a serious public health and infection control problems worldwide (1). For instance H. influenzaeis transmitted by respiratory droplets and may enter the bloodstream, where it can cause bacteraemia and disseminate to distant sites causing disorders such as otitis media, sinusitis, epiglottitis, bronchopneumonia, meningitis, septic arthritis and cellulitis resulting from invasion of the bloodstream, followed by localization of H. influenzae in these and other areas of the body (1,2). S. pneumoniaeis found in the nasopharynx of 5 to 10% of the healthy adults and 20% of healthy children. The organism attaches to the nasopharyngeal cells through interaction of bacterial surface adhesions. This normal colonization can become infectious in the Eustachian tube or nasal sinuses where it causes otitis media, sinusitis. The organism spreads to the bloodstream, initiating bacteraemia and is carried to the meninges, joint spaces, bones and peritoneal cavity and may result in meningitis, brain abscess, septic arthritis or osteomyelitis(2). The risk of pneumococcal infection is much increased in persons with impaired IgG specify the subset (e.g. IgG1,2,3, or4) and it possible molecular physiology activities in the management of pnemococcal synthesis, phagocytosis or defective clearance of pneumococci. In particular, the absence of functional spleen, through congenital asplenia, splenectomy, or sickle-cell disease predisposes one to a more severe course of infection (3).Bovine Tuberculosis and HIVare major diseases , which have also impacted on the economies of several countries. The morbidities and mortalities due to TB bacilli and HIV are well documented (3). Currently, there is a continuous spread of multi-resistant pathogens which have become a serious threat to public health and infection control practices worldwide (3). This problem has necessitated a search for new antimicrobial compounds from other sources including plants (4). It is expected that plant extracts showing target sites other than those used by antibiotics will be active against drug resistant pathogens as plant derived materials have provided the models for about 50% of western drugs (5,6). The enormous benefits of plants in the management of microbial infections are huge and knowledge of herbal formations made from roots, flowers, barks and their extracts are well documented (7). The great civilization of the ancient Chinese, Indians and North Africans provided written evidence of our ingenuity in utilizing plants for the treatment of a wide variety of diseases, including bacterial, viral, fungal and parasitic infections, as well as immunological disorders (8). It was reported (9,10), that about 25% of prescribed drugs in the world originated from plants and about 80% of the population in developing countries, including South Africa, rely on traditional plantsfor their primary health care needs. It was also reported (10), that Bangladesh had a rich and prestigious heritage of herbal medicines among the south Asian countries. More than 500 species of medicinal plants were estimated to be grown in Bangladesh and about 250 species of them were used in the preparation of traditional medicines and treatment of various diseases such as pneumonia, asthma, bronchitis, epiglottitis, septicaemia, meningitis, cellulitis, sepsis, arthritis, diabetes, cancer, inflammation anddiarrhoea diseases (11). Medicinal plants may be used for totally different ailments in different ethnic groups (12). It was reported (12), that about 70% of South Africans consult traditional healers and that 1% ofnurses are traditional healers. Antiviral substances have also been isolated from higher plants such as algae and lichens. The screening of plant extracts for antiretroviral activity was important because plant-derived anti-HIV compounds could inhibit the replication of the virus by interfering with one or more of the ten steps of the HIV replication cycle (13). Inspire of the therapeutic properties of plants derived products, their usefulness and efficacies will be severely compromised if they elicit adverse reactions on administration. This potent that their safety profiles must be guaranteed or that the side effects will be tolerable and not toxic to host cells. Therefore a determination of the cytotoxicity level of any medicinal plant will reveal its safety as a potential therapeutic agent. Consequently this study determined the cytotoxic activities of aqueous and methanolic extracts of nine South African medicinal plants in order to gauge their usefulness as potential candidates for eventual drug development.
2. Materials and Methods
2.1. Collection of Plant Material
- From June 2010 to June 2011, the leaves, bark, stem, roots and twigs of nine selected medicinal plants were collected from different sources in the Eastern Cape and Limpopo Provinces, South Africa, based on their ethnomedical application in the treatment of respiratory tract infections, guided by the information from traditional healers on the basis of their various uses. The plants (Table 1.) were identified and authenticated by Taxonomist (Immelman K.L.) in the Department of Botany, Walter Sisulu University, South Africa. Voucher specimens MI 001, MI 002, MI 003, MI 004, MI 005, MI 006, MI 007, MI 008 and MI 009 were deposited at the University herbarium.All the nine plants in Table 1 are used by traditional healers in South Africa, to treat diseases such as pneumonia, bronchitis, epiglottitis, asthma, septicaemia, cellulitis, sepsis, meningitis,and arthritis caused by respiratory tract opportunistic pathogens such as Haemophilusinfluenzae and Streptococcus pneumoniae.
2.2. Preparation of Plant Extracts
- Plant materials were washed with sterile distilled water, dried in an oven for 2h and cut into small pieces using a sharp knife and then air dried at room temperature for 7 days as previously described (14).The dried material (50g) was ground into a coarse powder using Macsalab mill (Model 200 LAB), Eriez, Bramley as previously described (14).The ground plant material was reduced to fine powder using an electric blender and the physiochemical components soaked in methanol (500ml) for 72h with frequent shakings, as previously described (14,15).The samples were suction filtered through Whatman No1 filter paper. The filtrate was evaporated to dryness under reduced pressure using a rotary evaporator, collected in 10ml of the solvent, placed in the tube and allowed to dry at room temperature.A stock solution of 0.2g/ml in dimethyl sulfoxide (DMSO) was made for each extract.All the extracts were kept at 4°C in the dark until they were further used.
2.3. Cytotoxic Screening of Nine Medicinal Plants Extracts
2.3.1. Cell Culture
- MAGI CCR5+ cells were used for cytotoxic screening of the nine medicinal plant extracts. All cell lines were purchased from ATCC, Manassas, VA 20108, USA. Cell lines were cultured in Advanced Modified Eagle’s Medium (DMEM) with 10% 5Mm L-glutamine (Gibco BRL) and grown at 37°C in a 5% CO2 humidified incubator (Thermo Fisher Scientific, Wakenyaku Co. Ltd, Japan). Cells were subcultured every 2 days after the confluent growth was observed.
2.3.2. MTT8 ASSAY 3-(Dimethylthiozole-2-yl-2,5-diphenyltetrazolium bromide)
- MAGI cells were seeded into two 96 well plates with 104 cells/well who was this determined?in 100µl of DMEM supplemented with 10% foetus bovine serum (FBS). 11µl of herbs was added into 2 wells of row B, with final concentration of 1/20. Another 11µl of mixture was removed from B to C and then to D, E, F, and 10µl was discarded from F. 100µl of medium was added into each well from B to G. After 48 h, cells were observed and 150µl of supernatant from each well was discarded and then 10µl of MTT was added into each well. The plates were incubated at 37°C for 4h. 100µl of stop solution was added into each well and OD570 was checked and then CC50 were determined, as previously reported (14, 15).
2.3.3. Detection of Anti-HIV-1 IIIB Activity of Nine Herbs Using MAGI Assay
- MAGI CCR5+ cells were seeded into two 96 well plates with 104 cells per well in 100µl of Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% foetal calf serum. Herbs in 50mg/ml DMSO, were serially diluted with complete medium, by adding 11µl of herbs into 2 wells of row B, with a final concentration of 1/20. 11µl of mixture was removed from B to C and then to D, E, F and 10µl discarded from F in flat bottomed microculture plates. The diluted virus was added 30 minutes later. HIV-infected (10ng of p24) or mock-infected MAGI CCR+cells were cultured with the extracts continuously present and no cell washing was performed throughout the culture. Cells were stained with chlorophenol red β-D-galactopyranoside (CPRG) as previously described (15). After 3 days of culture, the medium was removed and the cells were lysed with 100µl of phosphate buffered saline (PBS) containing 1% Triton X-100 for 30 minutes at room temperature. The cells were then incubated at 37℃ for 1h with 100µl of staining solution containing 0.01 M KH2PO4, 0.1 M K2PO4, 2mM MgCl2 and 10 mM CPRG. After 1h the results were observed under the microscope (14,15).
2.4. Virus Dilution
- IIIB virus (1/250) was used for screening of nine medicinal plant extracts in a 96 well microtitre plate, in which 100µl of the virus was added into each well. Cells were fixed with 1% formaldehyde and 0.2 % gluteraldehyde in Phosphate buffered saline (PBS) solution for 5 minutes at room temperature and then washed 3 times with Phosphate buffered saline. Cells were incubated in 50µl of 4mM potassium ferrocyanide, 4mM potassium ferricyanide, 2mM MgCl2 and 0.4mg/ml5-bromo-4-chloro-3-indolyl-2-D-galactopyranoside (X-gal) at 37℃ for 1h. The reaction was stopped by removing the staining solution and washing the cells twice with Phosphate buffered saline in each well. Blue cells were counted under a light microscope at X100 magnification (14,15).
2.5. Detection of Mycobacterium Tuberculosis Rv0679c Protein Activity of Nine Herbs Using Enzyme Linked Immunosorbent Assay (ELISA)
- The antigen (Rv0679c) was diluted with carbonate coating buffer (2µg/ml) and 50µl of the mixture was added into a 96 well ELISA microplate. The plate was then covered and incubated at room temperature for 24h. After 24h, the plate was washed once with phosphate buffered saline (PBS). The plate was then blocked with 250µl/well of the blocking buffer (PBS and 1% BSA), sealed and incubated at room temperature for 2h. The plate was washed 5 times with PBS and then different herb extracts were added and incubated at room temperature for 1h. After 1h the plate was washed once and the 1/20000 detection antibody (mAb5D4C2 conjugate) was added and the plate incubated at room temperature for 2h. After 2h the plate was washed and 1 drop of Vector Elite ABC reagent was added and the plate incubated at room temperature for 30 minutes. The plate was then washed and 100µl/well substrate (H2O2 and TMB reagent) was added and incubated in the dark room for 30 minutes. After 30 minutes 100µl of stop solution (2NH2SO4) was added and ODS was read immediately at 45nm. Inhibition control of the detection antibody (mAb8G10H2) and crude Human serum from TB positive patients was used (Cifuenteset al., 2010).
3. Results
3.1. Cytotoxic Effects of Nine Medicinal Plant Extracts (Herbs)
- The results on the Cytotoxic effects of the medicinal plant extracts tested are presented below.
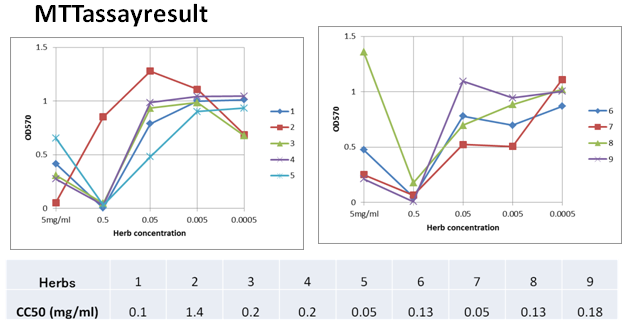 | Figure 1. 1-Vangueria infausta (Umviyo), 2- Cyanthulainculata (Isinama), 3-Croton grattismus (Umhlabakufeni), 4-Cassine transvaalensis (Ingwavuma), 5-Caparis tomentosa (Iquaningi), 6-Plumboza fingerleaf (Umpenduli), 7-Hypoxis hemerocallidea (Ilabatheka), 8-Lysium inerme (Umvuthwamini), 9-Dichrostachys cinetia (Umnukelambiba) |
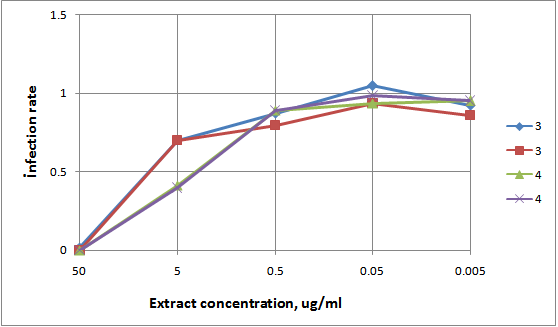 | Figure 2. Anti-HIV-1 IIIB activity of nine medicinal plant extracts using MAGI cells |
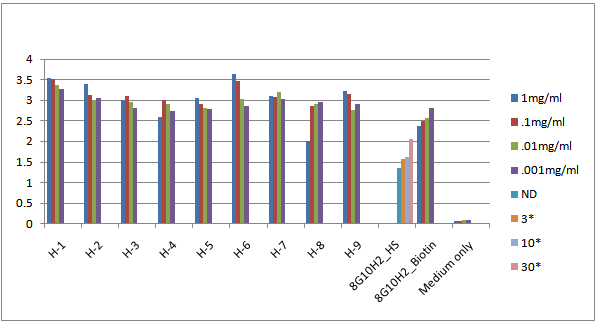 | Figure 3. 1.Vangueriainfausta (Umviyo), 2. Cyanthulainculata (Isinama), 3.Croton grattismus (Umhlabakufeni), 4.Cassinetransvaalensis (Ingwavuma), 5.Caparistomentosa (Iquaningi), 6.Plumbozafingerleaf (Umpenduli), 7.Hypoxishemerocallidea (Ilabatheka), 8.Lysiuminerme (Umvuthwamini), and 9. Dichrostachyscinetia (Umnukelambiba) |
3.2. Detection of Mycobacterium Tuberculosis Rv0679c Protein Activity of Nine Herbs Using Enzyme Linked Immunosorbent Assay (ELISA)
- Inhibition control of the detection antibody (mAb8G10H2) and crude Human serum from TB positive patients showed an inhibition and binding activity of 52% (figure 3) while 1mg/ml Herb-8 (Lysiuminerme, Umvuthwamini) showed an inhibition and binding activity of 29%. Other herbs showed no inhibition and specific binding against Mycobacterium tuberculosis Rv0679c antigen.
4. Discussion
- Microbial pathogens and diseases caused are enormous and are capable of impacting on several facets of education, economy and health care systems. Concerted and persistent efforts are therefore warranted in gauging the efficacies of plant derived antimicrobials as well as critical evaluations for their safety profiles.A safety profile is exemplified, among other factors and considerations, by cytotoxicity levels, although in-vitro results may not always simulate in –vivo conditions. However in-vitro cytotoxicitydeterminations could serve as one of the adjuncts in profiling justifications for clinical trials.In the present study, we do not have data on each of this plant extracts and their activities, no significance level determined, where are the data on binding of this compounds to the cells? The fact that the compounds bound to the cells does not signify their ability to kill the cells (cytotoxicity) leading to opsonisation; we should have the initial number of cells and the number of cells after the plant extract have been added to the wells. This assay would be best determinedwith a Flow cytometery, IEAs or by use of florochomes and not direct ELISA. medicinal plant extracts were screened for cytotoxic effects on MAGI cell lines. Of the nine plant extracts 2(22%), Croton grattismus and Lysiuminerme exhibited minimal toxicity on MAGI cells and 7(77.8%) exhibited 50% toxicity. Therefore (Crotongrattismus and Lysiuminerme) may be suitable for the treatment of infections caused by designated pathogens and this is consistent with a previous finding (15).In a similar study 2(22%) of the methanolic extracts exhibited anti-HIV1 IIIB activities. Human immunodeficiency virus (HIV) is currently one of the most serious infectious pathogens causing acquired immune deficiency syndrome (AIDS) and the observed anti-HIV activity as well as minimal toxicity of the plants offer promise for chemotherapy. Elucidation of the structures of the active compounds will be another focus of study. In a similar study methanolic extracts were screened against Mycobacterium tuberculosis (TB) and only one medicinal plant (Lysiuminerme) exhibited 29% toxicity. Tuberculosis is among the top three leading causes of death worldwide and it is aggravated by the increased susceptibility to the human immunodeficiency virus (HIV). The results obtained in this study had a slightly lower toxicities than the previous findings (1, 20)The results in this study show that the active ingredients of the plant parts are better extracted with methanol than other solvents, as were reported for Croton grattismus, Capris tomentosa, Cassine transvaalensis, Lysium inerme and Cyantula inculata (16,17,18,19).Both the methods and result sections are inadequate in content. They are not informative enough to bring out the phytochemical activities of the plant extracts.
5. Conclusions
- In this study, a systematic evaluation of cytotoxic activities of methanolic extractsmadefrom tested medicinal plantsshowed minimal toxicity of 22% on cell lines,therefore medicinal plantscould be used as natural antimicrobial therapeutic agents for the treatment ofdisorders caused by thedesignated pathogens.
ACKNOWLEDGEMENTS
- The authors wish to thank Walter Sisulu University (WSU), the National Research Foundation (NRF) of South Africa and the Medical Research Council (MRC) of South Africa for financial assistance. We are indebted to the technical staff of the Department of Medical Microbiology, Walter Sisulu University (WSU) for the technical assistance they provided during this research work. Special thanks go to the management and technical staff of the National Health Laboratory Services, Nelson Mandela AcademicHospital, Mthatha and The Laboratory for Emerging and Infectious Diseases, Tohoku University, Japan for the outstanding technical assistance provided during this research work.
ABBREVIATIONS
- BSA –Bovine serum AlbuminCPRG – β –D - galactopyranosideDMEM – Dulbecco’s Modified Eagles’s MediumDMSO – Dimethyl sulfoxideELISA – Enzyme Linked Immunosorbant AssayFBS – Foetus bovine serumIgG – Immunoglobulin GMTT–3-(4,5-Dimethylthiozol–2-yl)-2,5-diphenyltetrazolium bromideTB – Mycobacterium tuberculosis
References
| [1] | Corless CE, Guiver M, Borrow R, Edwards-Jones V, Fox AJ, and Kaczmarski EB (2001). Simultaneous detection of Neisseriaeminingitidis, Haemophilusinfluenzae andStreptococcus pneumoniae in suspected cases of meningitis and septicaemia using Real- Time PCR. Journal of Clinical Microbiology, 39: 1553-1558. |
| [2] | Akoachere TK, Ndip RN, Chenwi EB, Ndip LM, Njock TE (2002). Antibacterial effect of Zingiberofficinale and Garciniakola on respiratory tract pathogens. East African Medical Journal, 79: 11. |
| [3] | Ndip RN, Ntiege EA, Ndip LM, Nkwelang G, Akoachere TK, Akenji NT (2008). Antimicrobial resistance of bacterial agents of the upper respiratory tract of school children in Buea, Cameroon. The Journal of Health, Population and Nutrition, 26: 397-404. |
| [4] | Eloff JN, Lwalewa EO, Suleiman MN, Mdee LK (2009). Antifungal and antibacterial activities of different extracts of Harunganamadagascariensis stem bark. Journal of Drug Assessment, 7: 64-78. |
| [5] | Rajendran NK and Ramakrishnan J (2009). In vitro evaluation of antimicrobial activity of crude extracts of medicinal plants against multi-drug resistant pathogens. Journal of Medical Microbiology 2: 97-101. |
| [6] | Uyub AM, Nwachukwu IN, Azlan A, Fariza SS 2010. In-vitro antibacterial activity and cytotoxicity of selected medicinal plant extracts from Penang Island, Malaysia on Metronidazole – resistant – Helicobacter pylori and some pathogenic bacteria. Ethnobotany Journal of Research and Applications, 8: 095-106. |
| [7] | Wanjala CCW and Majinda RRJ (2001). Two novel glucodienoid alkaloids from Erythrinalatissima seeds. Journal Natural Products, 63: 871-73. |
| [8] | Ramalivhana JN, Moyo SR, Obi CL (2010). The possible role medicinal plants in tackling resistance microbial pathogens in Limpopo Province, South Africa. Journal of Medicinal PlantsResearch,4: 999-1002. |
| [9] | Uddin SJ, Grice ID, Tiralongo E (2009). Cytotoxic effects of Bangladeshi Medicinal Plant Extracts. Journal of Evidence- Based Complementary and AlternativeMedicine, 2011: 1093. |
| [10] | Steenkamp V and Gouws MC (2006). Cytotoxicity of six South African medicinal plant extracts used in the treatment of cancer. South African Journal of Botany,72: 630-633. |
| [11] | George S, Bhalerao SV, Lidstone EA, Ahmad IS, Abbasi A, Cunningham BT, Watkin KL (2010). Cytotocity screening of Bangladeshi Medicinal Plant Extracts on pancreatic cancer cells. Journal of Complementary and AlternativeMedicine,10: 52. |
| [12] | Samie A, Housein A, Lall N, Meyer JJM (2009). Crude extracts of purified compounds from Pterocarpusangolensis and the essential oil of Lippia against selected bacteria and Entamoebahistolytica. Annals of Tropical Medicine and Parasitology, 103: 427-439. |
| [13] | Abonyi DO, Adikwu MU, Esimone CO, Ibezim EC (2009). Plants as sources of antiviral agents. African Journal Biotechnology, 8: 3989-3994. |
| [14] | Obi CL, Ramalivhana J, Samie A, Igumbor EO (2007). Prevalence, Pathogenesis, Antibiotic susceptibility profiles and In-vitro activity of selected medicinal plants against Aeromonas isolates from stool samples of patients in the Venda region of South Africa. The Journal ofHealth, Population and Nutrition, 25: 428-435. |
| [15] | Theo A, Masebe T, Suzuki Y, Kikuchi H, Wada S, Obi CL, Bessong OP, Usuzawa M, Oshima Y, Hattori T (2009). PeltophorumAfricanun, a traditional South African medicinal plant, containing an AntiHIV-1 constituent Betulinic Acid. Tohoku Journal of Experimental Medicine,217: 93-99. |
| [16] | Suleiman MM, McGaw LJ, Naidoo V, Eloff JN (2010). Detection of antimicrobial compounds by bioautography of different extracts of leaves of selected South African tree species. African Journal of Traditional, Complementary and Alternative Medicines, 7: 64-78. |
| [17] | Matsuba T, Suzuki Y, Tanaka Y (2007). Association of the Rv0679c protein with lipids and carbohydrates in Mycobacterium tuberculosis and Mycobacterium bovis BCG. Archieves of Microbiology.187: 297-311. |
| [18] | Buwa LV and Afolayan AJ (2009). Antimicrobial activity of some medicinal plants used for the treatment of tuberculosis in the Eastern Cape Province, South Africa. African Journal of Biotechnology,8: 6683-6687. |
| [19] | Bisi-Johnson MA, Obi CL, Hattori T, Oshima Y, Li S, Kambizi L, Eloff JN, Vasaikar SD (2011). Evaluation of the antimicrobial and anticancer activities of some South African medicinal plants. Journal of Complementary and Alternative Medicine, 11: 14. |
| [20] | Cifuentes DP, Ocampo M, Curtidor H, Vanegas M, Forero M, Patarroyo ME, Patarroyo MA (2010). Mycobacterium tuberculosis Rv0679c protein sequences involved in host-cell infection: Potential TB vaccine candidate antigen. Journal ofBioMedical Central, 10: 109. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML