-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Microbiology Research
p-ISSN: 2166-5885 e-ISSN: 2166-5931
2012; 2(6): 170-175
doi: 10.5923/j.microbiology.20120206.03
Evaluation of Streptomyces Aureofaciens and Rhodotorulaglutinis Against Ochratoxin A Producing Aspergillusnigrin Grapevines
Wafaa M. Haggag , Abdall A. M.
Department of Plant Pathology, National Research Centre , Dokki, Egypt
Correspondence to: Wafaa M. Haggag , Department of Plant Pathology, National Research Centre , Dokki, Egypt.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
OchratoxinA (OTA) is a mycotoxin, produced by filamentous fungi, toxic to humans and animals and naturally found in a wide range of different agricultural products, includingfruits (grapevines).Members of Aspergillussection Nigri(black aspergilli) are mainly responsible for OTA accumulation in seeds. This research investigate the biological control of ochratoxin A (OTA) production by A. niger, using, that proved variable antagonistic activity against A. niger. A general inhibition effect was observed with tested Rhodotorulaglutinis strain followed by Streptomyces aureofaciens. A field experiment conducted during 2011 and 2012 vintages in a vineyard indicated that yeast spraying was found to be very efficient for reducing A. niger development. Grape berries sprayed with either with Streptomyces aureofaciens orRhodotorulaglutinis. Rhodotorulaglutinis completely inhibited OchratoxinAproducingA. niger for 40 days of storage.
Keywords: Aspergillusniger,Mycotoxin, Ochratoxin A.
Cite this paper: Wafaa M. Haggag , Abdall A. M. , "Evaluation of Streptomyces Aureofaciens and Rhodotorulaglutinis Against Ochratoxin A Producing Aspergillusnigrin Grapevines", Journal of Microbiology Research, Vol. 2 No. 6, 2012, pp. 170-175. doi: 10.5923/j.microbiology.20120206.03.
Article Outline
1. Introduction
- Growing mould may produce toxic secondary metabolites, such as mycotoxins. Among hundreds of fungal secondary metabolites are mycotoxins which include aflatoxins (AFL) and ochratoxin A (OTA)[10]. They are of major health concern for humans and domestic animals[12]. Mycotoxins can enter into the human food chain directly through foods of plant origin and indirectly through foods of animal origin[8]. The International Agency for Research on Cancer classified OTA as a possible carcinogen to humans (group 2B) (International Agency for Research on Cancer (IARC) [7].Many types of food products in the markets have been reported to be contaminated with OTA. These include tree nuts, peanuts, figs, melon seed, pumpkin seed, sesame seed, sunflower seed, lotus seed, corn seed, red pepper, white pepper, mixed spices, rice, corn, mixed cereals, chilies, and copra[18]. The contamination of grapes and their by-products by OTA has emerged as a big problem for the health risk related to the consumption of such products by human beings[10]. The European Unionhas introduced limits (2 μg L−1) for OTA in grape products in order to minimize exposure to OTA in the diet (EC Regulation No 1881/2006). Ochratoxin A is produced during the infection on grapes in vineyards by toxigenic species of black aspergilli belonging to Aspergillus section Nigri, in particular A. niger[10].Biological control is being increasingly considered by the scientific community as a reliable alternative to pesticide utilization in field and in post-harvest. A wide array of organisms have been tested for biologicalcontrol of aflatoxin contamination including bacteria, yeasts,actinomycetes, algae[13]. Biological control offers an alternative for the chemical control of pre and post harvest diseases offruits which includes use of plant products and antagonistic organisms. Many species of actinomycetes,especially those belonging to the genus Streptomyces , are well known as biocontrol agents that inhibitor lyse several airborne plant pathogenic fungi[17]. It is well known thatStreptomyces sp. can produce industrially useful compounds, notably wide spectrum of antibiotics, assecondary metabolites, and continues to be screened for new bioactive compounds[14]. From these species, Streptomyces aureofaciensantagonist, is promising candidates as bioprotectants.Recently, an antagonisticyeast strain of Rhodotorula glutinis have been reported as an effective biocontrol agent against postharvest decay of apples[16], pears [20], strawberries[21] and oranges[22].The main objective of this study is to prevent or inhibit the growth of ochratoxin producing A. nigerusing biological method in grapevine.
2. Materials and Methods
2.1. Collection of Grape Samples and Isolation of Aspergillusniger
- Grape samples were collected at harvesting stage from Khatatbaand Noubariaregions, Egypt. At harvest, five berries were taken randomly from each bunch and they were surface decontaminated using a 0.1% sodium hypochlorite solution for 30 s followed by two rinses with sterile-distilled water.The samples were plated on Potato dextrose agar (PDA, Merck) dishes containing streptomycin 50 mg L−1 (Merck). Plates were incubated at 25℃ for 7 days. The fungal colonies were picked up and transferred to PDA slants for further studies. The initial identification of isolates of Aspergillusspp. was achieved through macroscopic and microscopic observation with the aid of guidelines[8].
2.2. Determination of Ochratoxigenic Potential OfAspergillus IsolatedOTA Production
- OTA production of the isolated Aspergillusnigerwas determined on PDA medium. Inoculateswere prepared by growing the strains on PDA medium at 25℃ for 5 days. Conidiasuspensions of each isolate were prepared in a physiological water (0.85% NaCl) containing1% Tween 80 and adjusted to 106conidia/ml. The adjustedsuspension of 5 μl was dropped atthe centre of PDA medium plate. The plates were incubated at 25℃ for 7 days.
2.3. Extraction And Quantification of OTA from Culture Medium
- After 10 days of incubation, 3 agar plugs of 5 mm in diameter were removed from middle areaof the colony. The plugs were weighed and extracted with methanol/formic (25:1) undersonication for 15 min. After evaporating the solvent under a nitrogen stream at 40℃, the driedextracts were re-suspended in the mobile phase of HPLC (acetonitrile:deionizedwater:aceticacid, 45.5:49.5:1). The filtered extract was analyzed for OTA determination by HPLC with C18 (254.6 mm, 5 μm) and fluorescence detector by using the method of Dachoupakanet al[4].
2.4. Biological Control
- Streptomyces aureofaciens and Rhodotorula glutinis were previously isolated from grape leavesgrownin Noubaria regions, Egypt and identified in Plant Pathology Department, National Research Centre, Egypt. Cultures were grown and maintained on solid starch medium at 28℃ and Nutrient agar medium, respectively.Streptomyces aureofaciens and Rhodotorula glutinis were grown in starch nitrate and Nutrient agar medium for 7 and 4 days,respectively. Aliquots (100 μl) of each cell free extract which was previously extracted by milling the cells in sterile saline were applied on the holes, and agar diffusion method was applied. After incubation for 7 days, the diameters of inhibition zones were measured.OTA were determined in inhibition regions as previous above.
2.5. Field Experiment
- Experiments were conductedunder natural conditions during 2011 and 2012 seasons using Film cultivar (Vitisvinifera L.cv. Felam) grown in a private vineyard at El-Khatatba, Monofiagovernorate on eightyearsold,.The vines were placed at 1.5 m (between vines in the row) x 2.75 m (between rows). The vineyard experiments were arranged in a randomized complete block design with four replications for each variant, four vines for each replicate.Treatments were applied twice at 20-d intervals on grape vines in on-year, four weeks before harvest. All blocks were harvested separately by hand at grape maturity. Three weeks before the expected technological harvest time, vine rows were sprayed with a suspension of Streptomyces aureofaciensor Rhodotorula glutiniscells at 105 cells | ml. Sterile water was sprayed uniformly on the corresponding blocks. At the time of harvest, about three different lots of 100 grape berries were aseptically randomly harvested in each block, rinsed with sterile water in the presence of Tween 80 (an anionic detergent) and plated on selective culture media to estimate fungus surface contaminations. For estimating fungi contamination inside the grape berries, the previous berries were surface-disinfected with sodium hypochlorite solution (1%) for 1 min, rinsed in sterile distilled water three times rinsed two more times with sterile water and aseptically hand-crushed in sterile small poly bags. The resulting juices were then plated on selective culture media to estimate internal invasive contamination of grape berries by fungus.Ochratoxin A determination.Ochratoxin A level in the grape was analyzed asprevious above.The total yield (Kg) per vine was recorded.At harvest date (the 1st week of June) some measuring of vegetative growth and the yield per vine was recorded in the term of weight (in kg).
2.6. Post–harvest Infection
- Grapes berries from harvest were superficially sterilized with sodium hypochlorite at 2% v/v for 2 minutes, rinsed with sterile distilled H2O and incubated in container at 30℃ after superficial inoculation by 20μl of conidia suspension (2.5 CFU/μl) prepared from OTA producer strain of A. niger. Four different experiments have been performed during 40 days of post-harvest storage: at harvest, after 15, 25 and 40 days of conservation. . Inoculated and un-inoculated berries samples were collected. Ochratoxin A level in the table grape was analyzed asprevious above.
2.7. Statistical Analysis
- For data analysis, the statistical computer application package SPSS 10.0 will be employed. Data will be subjected to analysis of variance (ANOVA) and the means will be compared for significance using Duncan's Multiple Range Test (DMRT; P = 0.05).
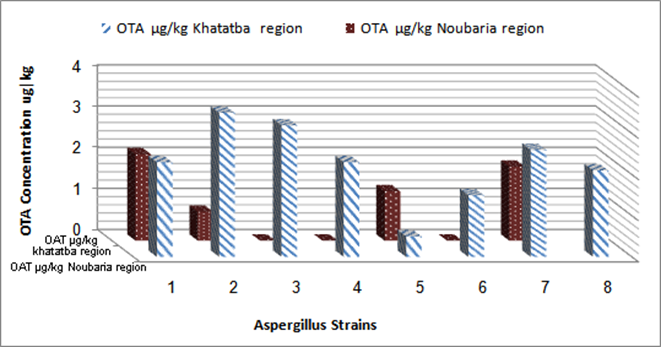 | Figure 1. Ochratoxin A (μg/kg) produced by Aspergillusniger isolated from grapevines grown in Khatatbaand Noubaria region |
3. Results
3.1. Determination of Ochratoxigenic Potential of AspergillusIsolatedOTA Production
- Eleven strainsof the 15 isolates of Aspergillusnigerwere ochratoxin producers. Aspergillus strains isolated from grapevines grown in Khatatbaregion(Fig. 1). Sinceconcerning the 8strains isolated from grape fruits, were ochratoxinogenic with levels ranging from 0.5to 3.5 μg/kg. The incidence of ochratoxigenic isolates was very low, and the levels of OTA produced by A. nigerisolated fromNoubaria region, were quite similar( 0.2- to 2.34μg/kg).
3.2. In Vitro, Antagonism Study of Biocontrol Agents on the Growth of Ochratoxin Producing A. Niger
- In this experiment, Streptomyces aureofaciens and Rhodotorula glutinis were tested to control the growth of the producer Aspergillus of ochratoxin (Table 1). Five Aspergillus strains were selected according to produce the Ochratoxin A.The well diffusion method was used to determine the anti-fungal activity ofboth isolates. Rhodotorulaglutinis was the best isolate used that revealed the highest antifungal activity against A. niger. However, Streptomyces aureofaciensshowed high effect against Aspergillusniger growth. The same results were foundin reducing of Ochratoxin A produced by Aspergillusniger strains (Table 1). Rhodotorulaglutinis was the best isolate used to reduceOchratoxinA .Streptomyces aureofacien sshowed high effect in reducing Ochratoxin A.
3.3. Field Experiments
- Fourweeks before harvest time, vine rows were sprayed with a suspension of Streptomyces aureofaciens and Rhodotorulaglutinis. Both microorganismswere effective in reducingA. nigercontamination to low levels in grape berriescv. Felamgrown inKhatatbaregion(Table 2). Rhodotorulaglutinis completely inhibited the growth ofA. Nigerin grape berriesin both seasons. Streptomyces aureofaciens was also effective in reducingA. niger contaminationto low levels in grape berries compared with untreated control in both seasons.OchratoxinAwas completely inhibited in the present of eitherStreptomyces aureofaciens or Rhodotorula glutinis in compared with untreated control in both seasons.The efficacies of spraying of Streptomyces aureofaciens and Rhodotorula glutinis to improvegrapevine cv. Flamyield were determined in. Kalubiaregionin season 2011 and 2012. Results of Fig. 2 indicated that, a general increase yield / vine was observed with Rhodotorula glutinis than either Streptomyces Aureofaciens and untreated control.
| |||||||||||||||||||||||||||||||||||||
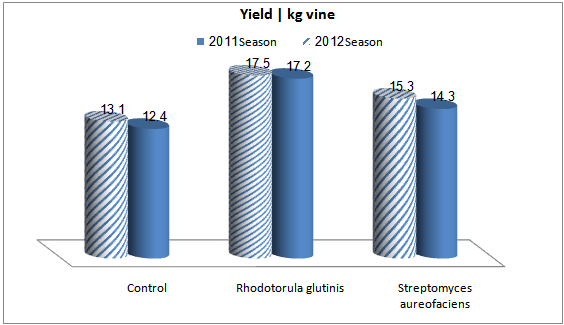 | Figure 2. Vine yield of mature bunches of "Flam" grapevine as affected by spraying Streptomyces aureofaciens orRhodotorulaglutinis |
3.4. Harvest Stage
- Effect of pretreatment with biocontrol agents on protection of table grape berries during storage from fungal infection (for 40 days) wasevaluated (Fig. 3 A and B). During storage time, the table grape berries become more susceptible to theattack by Aspergillusfungus stored for 40 days then uninoculated control (Fig.3 A). Hence, the development of this fungus was much rapid reaching high level of infection after 40 days of post-harvest cold storage. On contrast was found in grape berries sprayed with either with Streptomyces aureofaciens or Rhodotorula glutinis. Rhodotorula glutinis completely inhibited the growthofA. nigerfor 40 days . However, Streptomyces aureofaciens was also effective in delaying and reducingA. nigercontamination to low levels in grape berriesafter storage 40 days. The same results were foundinreducing of Ochratoxin A produced by Aspergillusniger strainsfor 40 days then uninoculated control (Fig. 3B). Rhodotorula glutinis was the best isolate used to prevent Ochratoxin Aproduction .Streptomyces aureofaciens showed high effect in reducingOchratoxin A during storage.
4. Discussion
- In the present study, biological control of ochratoxin A producing A. nigerusing antagonist bacteria and yeast was applied through screening program using soliedcultures. The program showed that Rhodotorula glutinis isolate was effective against the growth of ochratoxin producing A. niger. Recently, an antagonistic yeast strain of Rhodotorula glutinishave been reported as an effective biocontrol agent (in vivo) against postharvest decay of fruits and vegetables[20 & 11]. In previous studied we found that Rhodotorulaglutinisas protective agents against toxic effect of aflatoxin B1 in mice[6]. Similarly, yeast speciesisolated from grape berries, namely Issatchenkiaorientalis, Metschni kowiapulcherrima, Issatchenkiaterricola, and Candidainco mmunis havebeen reported to reduce colonization of grapeberries by A. carbonariusand A. niger, the mainspecies responsible for the accumulation of OTA in grapes[2]. However, Streptomyces aureofaciens showed high effect inreducing of Ochratoxin A produced by Aspergillusniger strains.Actinomycetes are known as producers of antibiotics and other biologically active substances with high commercial value such as vitamins, alkaloids, plant growth factors, enzymes and enzyme inhibitors[2]. They are the prolific producers of antibiotics and other industrially useful secondary metabolites[15]. Many species of actinomycetes have the capacity to inhibit pathogenic fungi[5]. Among the most known genus, are Streptomyces.
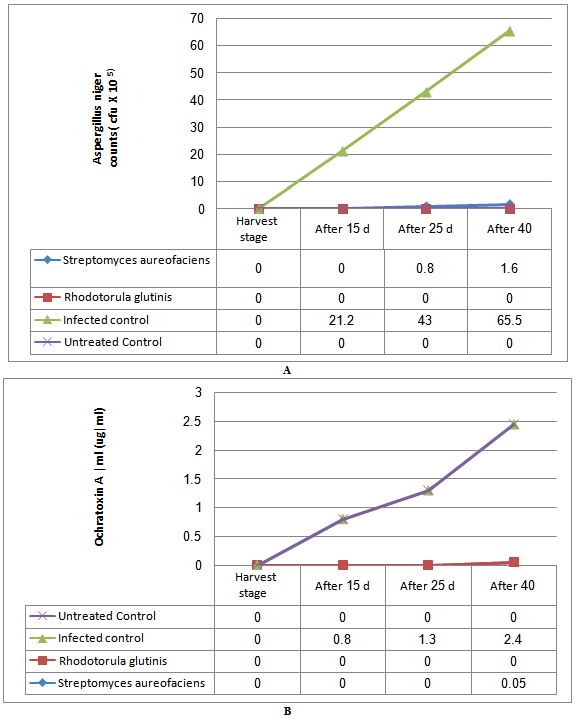 | Figure 3. Mean of infected grapes Felamcultivar with A. niger(c) and OchratoxinA(B), after 0 (A), 15 (B), 25 (C) and 40 (D) days of postharvest cold storage during different incubation periods |
References
| [1] | Baltz RH, Roundtable MF (2006). Is our antibiotic pipeline unproductive because of starvation, constipation or lack of inspiration? J. Ind. Microbiol. Biotechnol. 33: 507-513. |
| [2] | Bleve G., Grieco F., Cozzi G., Logrieco A., Visconti A. (2006): Isolation of epiphytic yeasts with potential for biocontrol of Aspergilluscarbonariusand A. niger on grape. International Journal of Food Microbiology, 108: 204–209. |
| [3] | Chand-Goyal, T.; Spotts, R.A. 1996. Postharvest biological control of blue mold of apple and brown rot of sweet berry by natural saprophytic yeasts alone or in combination with low doses of fungicides.Biological Control 6: 253-259. |
| [4] | Dachoupakan, C., Ratomahenina, R., Martinez, V., Guiraud, J.P., Baccou, J.P. and Schorr-Galindo, S. (2009). Study of the phenotypic and genotypic biodiversity of potentially ochratoxinogenic black aspergilli isolated from grapes. International Journal of Food Microbiology, 132:14-23. |
| [5] | Dahiya N, Tewari R, Hoondal GS (2006). Biotechnological aspects of chitinolytic enzymes. Appl. Microbiol. Biotechnol. 71(6): 773-782. |
| [6] | Inas S. Ghaly, M.M. Hassanane, E.S.Ahmed, W. M. Haggag S., A. Nada and I. M. Farag (2010).Cytogenetic and Biochemical Studies On the Protective Role of Rhodotorulaglutinis and its Autoploidy Against the Toxic Effect of Aflatoxin B1 in Mic. Nature and ScienceUSA ; 8(2): 7-23 |
| [7] | International Agency for Research on Cancer (IARC). Some Naturally Occurring Substances:Food Items and Constituents. Heterocyclic Aromatic Amines and Mycotoxins, World HealthOrganization: Lyon, France, 1993; Volume 56. |
| [8] | Klich, M.A. Identification of Common Aspergillus Species; Centraalbureauvoor Schimmelcultures: Utrecht, The Netherlands, 2002. |
| [9] | Kovacs M (2004). Nutritional health aspects of mycotoxins. Orvosi.Hetilap. 145(34): 1739-1746. |
| [10] | Leong, S.L.; Hocking, A.D.; Scott, E.S. species producing ochratoxin A: Isolation from vineyard soils and infection of Semillon bunches in Australia. J. Appl. Microbiol. 2007, 102,124–133. |
| [11] | Malisorn, C. and Suntornsuk, W. (2008): Optimization of β-carotene production by Rhodotorulaglutinis DM28 in fermented radish brine. Bioresource Technology, 99(7):2281 - 2287. |
| [12] | Miller D (1994). Review: fungi and mycotoxins in grain: implications for stored product research. J. Stored Prod. Res. 30: 1-16. |
| [13] | Mishra, H. N., & Das, C. (2003). A review on biological control and metabolism of aflatoxin. Critical Reviews in Food Science and Nutrition, 43(3), 245–264. |
| [14] | Mukherjee G. and Sen, S.K. 2006. Purification, Characterization, and antifungal activity of chitinase from Streptomyces venezuelaeP10.CurrMicrobiol. 53:265-9. |
| [15] | Nermeen AE, Gehan MA (2006). Antagonistic effect of marine Nocardiabrasiliensisagainst the fish pathogen Vibrio damsela: Application ofPlackett-Burman experimental design to evaluate factors affecting theproduction of the antibacterial agent. Int. J. Oceans and Oceanogr. 1:141-150 |
| [16] | Qin, G. Z., Tian, S. P., Liu, H. B., &Xu, Y. (2003). Biocontrol efficacy of three antagonistic yeasts against Penicilliumexpansum in harvested apple fruits. Acta Botanica Sinica, 45, 417–421. |
| [17] | Sousa C, Soares A.C. and Garrido, M. 2008. Characterization of Streptomycetes with potential to promote plant growth and bio-control.Sci. Agric. (Piracicaba, Braz.), 65: 50-55. |
| [18] | Wilson DM (2002). In: Mycotoxins. DeVries JW, TrucksessMW , Jackson LS, editors. Kluwer Academic/ Plenum Publishers; New York.Food Saf. pp. 5-17. |
| [19] | Yoshiteru H (2007). Development of novel expression systems for actinomycetes.Actinomycetologica. 21: 70-75. |
| [20] | Zhang, H. Y. ; Wang, S. Z. ; Huang, X. Y. ;Dong, Y. and Zheng X. D. (2008): Integrated control of postharvest blue mold decay of pears with hot water treatment and Rhodotorulaglutinis. Postharvest Biology and Technology, 49, 308-313. |
| [21] | Zhang, H. Y., Wang, L., Dong, Y., Jiang, S., Cao, J., &Meng, R. J. (2007). Postharvest biological control of gray mold decay of strawberry with Rhodotorulaglutinis. Biological Control, 40, 287–292. |
| [22] | Zhang, H. Y., Wang, S. Z., Huang, X. Y., Dong, Y., &Zheng, X. D. (2008). Integrated control of postharvest blue mold decay of pears with hot water treatment and Rhodotorulaglutinis. Postharvest Biology and Technology, 49, 308–313. |
| [23] | Zheng, X. D., Zhang, H. Y., & Sun, P. (2005). Biological control of postharvest green mold decay of oranges by Rhodotorulaglutinis. European Food Research and Technology, 220, 353–357. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML