-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Microbiology Research
p-ISSN: 2166-5885 e-ISSN: 2166-5931
2012; 2(5): 145-151
doi: 10.5923/j.microbiology.20120205.06
Purification and Characterization of Chitinase Produced by Endophytic Streptomyceshygroscopicus Against Some Phytopathogens
Wafaa M. Haggag 1, ElhamG. Abdallh 2
1Department of Plant Pathology, National Research Center, Dokki, Cairo, Egypt
2Biotechnology Unit, Ain Sham University
Correspondence to: Wafaa M. Haggag , Department of Plant Pathology, National Research Center, Dokki, Cairo, Egypt.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Microorganisms, which secret a complex of mycolytic enzymes are considered to be possible biological control agents of plant diseases. Since Chitinases are digestive enzymes that break down glycosidic bonds in chitin. In this present study, an industrial enzyme chitinase produced by endophyticStreptomyceswas purified and its antifungal activity was investigated against phytopathogens i.e. Rhizoctoniasolani,Fusariumoxysporum, Alternaria alternate, Aspergillusniger, Aspergillusflavus, Sclerotiniascleotiorum, Phytophthoraparasiticaand Botrytis cinerea.A chitinasewas produced byendophyticStreptomyceshygroscopicus in cultures containing chitin as the sole substrate that degrade chitin in 0.9 mm zone of clearance. The purification steps included ammonium sulfate precipitation, with columns of DEAE-Sepharose anion exchange chromatography and Sephacryl S-400 high resolution gel chromatography. The method gave a 5.4 fold increase of the specific activity and had a yield of 18%. The molecular weight of the chitinase was found to be around 80.8, 78 and 76 kDa by sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) technique. Chitinase was optimally active at pH of 6.0 to7.0 and at 30℃. The chitanase were found to inhibit the growth of all phytopathogenic fungi.
Keywords: Antifungal Activity ,Chitinase, PlantPhytopathogens, Streptomyceshygroscopicus
Article Outline
1. Introduction
- Fungal phytopathogens pose serious problems worldwide in the cultivation of economically important plants. Chemical fungicides are extensively used in current agriculture. However, excessive use of chemical fungicides in agriculture has led to deteriorating human health, environmental pollution, and development of pathogen resistance to fungicide. Because of the worsening problems in fungal disease control, a serious search is needed to identify alternative methods for plant protection, which are less dependent on chemicals and are more environmentally friendly. Microbial antagonists are widely used for the biocontrol of fungal plant diseases[1]. Endophytic micro-organisms have received considerable attention for their potential as biocontrol agents of fungal plant pathogens. Varied enzyme production may result in new biochemical characteristics and in part be responsible for the inherent biodiversity of endophytic micro-organisms. Among the lytic enzymes evaluated as a source of biocontrol agents, chitinases have been studied largely because these enzymes are produced by a variety of endophytic micro-organisms[2]. Endophyticactinomycetes have been cited as promising biocontrol agents, either acting directly on fungal cell walls or initiating increased plant responses against disease. In addition, research indicates that both mechanisms operate to control fungal pathogens[3]. Enzyme technology is an interdisciplinary field, and enzymes are routinely used in many environmental-friendly industrial sectors. With the advancement in biotechnology especially in the area of genetics, protein engineering, developments in bioinformatics, and the availability of sequence data have opened a new era of enzyme applications in many industrial processes[4]. Chitinase, a group of enzymes capable of degrading chitin directly to low molecular weight product and found in a broad range of organisms, including bacteria (Bacillus, Aeromonas, Pseudomonas, Serratia, Enterobacter, Actinomycete), fungi (Trichodermaand Aspergillus), and higher plants, insects, crustaceans, invertebrates and some vertebrates[5]. Chitinases have received increased attention due to their wide range of biotechnological applications, especially in the biocontrol of fungal phytopathogens[6]. Chitinasehave been implicated in plant resistance against fungal pathogens because of their inducible nature and antifungal activities in vitro. In viruses, chitinases are involved in pathogenesis[4]. Due to multiple applications of chitinases in biocontrol, waste management, medicine and biotechnology they become interesting enzymes for study .In the present study, chitinase production by endophyticactinomycetes, Streptomyceshygroscopicus and their anti-fungal activities agains tphytopathogens. Rhizoctoniasolani, Fusariumoxysporum, Alternaria alternate, Aspergillusniger, Aspergillusflavus, Sclerotiniascleotiorum, Phytophthoraparasitica and Botrytis cinerea was evaluated
2. Materials and Methods
2.1. Micro-organism Strains and Growth Conditions
- Streptomyceshygroscopicuswas previously isolated from peanut plants and identified in Plant Pathology Department, National Research Centre, Egypt. Culture was grown and maintained on solid starch medium[7]at 28℃. The fungal pathogens Rhizoctoniasolani , Fusariumoxysporum, Alternariaalternate, Aspergillusniger, Aspergillusflavus, , Sclerotiniascleotiorumand Botrytis cinereawere isolated originally from different naturally diseased plants collected from different agricultural fields. The isolated fungi were grown on potato dextrose agar (PDA) (Difco, USA) plates and incubated at 28℃ for 4 to 6 days. Purification of the resulting isolates was done using the hyphal tip or single spore techniques to obtain them in pure cultures; the detected isolates were then transferred into slant of PDA and kept at 4℃ for further studies. Pure cultures of the isolated fungi were identified according to the cultural properties, morphological and microscopical characteristics of each fungus[8]. Antagonistic activity assay: Dual culture assay was performed to assess the potential biocontrol activity of Streptomyceshygroscopicus against pathogenic fungi. In the dual culture assay, conidial suspension of each pathogen and bacterial suspension of Sterptomyceswere streaked on the surface of LB agar at a distance of 4 cm. The growth of both was examined daily for the formation of inhibition zone and growth reduction.
2.2. Quantitative Determination of Extracellular Chitinase Activity
- Sterptomyces was grown in ISP-2 broth[9]. with continuous shaking at 150 rpm at 27 ℃for 10 days. Cell-free supernatants were collected at 1-day intervals by centrifugation at 8,000 rpm for 20 min at 4 ℃. Cell pellets were dried at 80 ℃ and weighed to determine the growth. Chitinase activity was quantitatively determined by measuring the reducing end group of N-acetylglucosamine (NAG)that degraded from colloidal chitin as substrate[10]. The estimation of chitinase activity was carried out by the procedure described by Wang et al.[11]. Purification of chitinase: The proteins from the culture were precipitated by ammonium sulphate (75%). The precipitate was collected by centrifuging at 8,000 × g for 20 min, and resuspended in an acetate buffer (50 mM, pH 5.0). It was dialyzed against the same buffer and freeze-dried. The sample was then loaded on a preequilibrated DEAE-cellulose column (2 × 20 cm), and washed with the acetate buffer. The proteins were eluted in a stepwise gradient on NaCl (0-1.0 M) at a flow rate of 24 ml/h. Fractions of 3 ml were collected, and the absorbance was read at 280 nm in a spectrophotometer. The fractions with chitinase activity were combined, dialyzed against the acetate buffer, and concentration by lyophilization. The concentrated sample was passed through a Sephadex G-100 column (2 × 40 cm) and eluted with the acetate buffer at the rate of 15 ml/h. The fractions of 3 ml were collected, and the absorbance and chitinase activity were measured.Chitinase activity measurements A fluorometric assay was used to determine chitinase activity using 4-methylumbelliferyl- N,N',N''-chitotriose (Sigma, St. Louis, USA) as a substrate. The amount of 4 methylumbelliferone (4-MU) released was measured spectrofluorometrically by using a fluorescence spectrophotometer (F-4500, Hitachi) (excitation 390 nm and emission 450 nm). One unit (U) of chitinase activity was defined as the amount of enzyme required to release one μmol of 4-MU per min at 37℃.SDS-PAGE analysis :Sodium dodecyl sulphatepolyacrylamid gel electrophoresis (SDS-PAGE) was performed according to Laemmli procedure[12]. using the Mini- Protean II apparatus (Bio-Rad, Herculus, USA). A 10% separating gel containing 0.01% glycol chitin as the substrate of chitinase was used to detect chitinase activity. After electrophoresis, proteins in the gel were renatured in 0.1 M sodium acetate buffer (pH 5.0) containing 1% Triton X-100 at 37℃. Subsequently, the gel was stained with 0.01% Calcofluor White M2R (Sigma, St. Louis, USA) in 0.5 M Tris-HCl (pH 8.9) and examined for the chitinolytic bands under UV transilluminator[13]. In another gel, proteins were stained with Coomassie Brilliant Blue G-250.Protein concentration was determined by Bradford’s method[14]using bovine serum albumin as standard.
2.3. The studies of chitinases characterization Determination of optimum temperature and pH
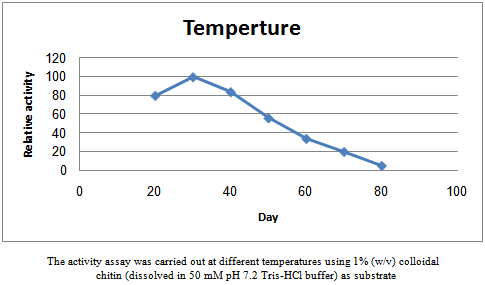
- The reaction was carried out at temperatures ranging from 20 to 80℃ and the chitinaseactivity at different temperatures were measured to find the optimum temperature. A similar method was applied at varying pH levels from 3.0 to 8.0 to determine the optimum pH.
2.4. Inhibition of Fungal Growth by the Purified-Enzyme Extract
- A spore suspension of pathogenic fungiwas uniformly spread on plates of PDA (potato dextrose agar). Discs that were soaked with the purified enzyme extract (5 U, 10 U) were laid on the seeded plates; the control was disc-soaked in boiled-enzyme extract. Fungal growth was observed over 2 d of incubation at 30℃.
2.5. Statistical Analysis
- All data (chitinase production and antagonistic isolates) were analysed for significance (P < 0.01) using the SAS software package.
3. Results
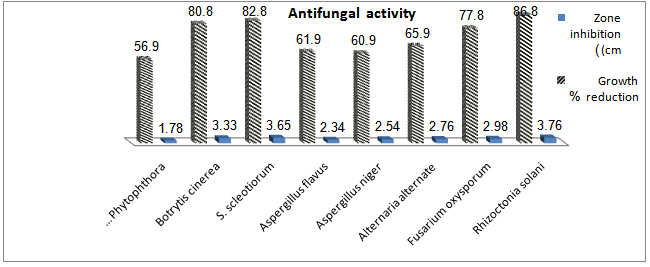
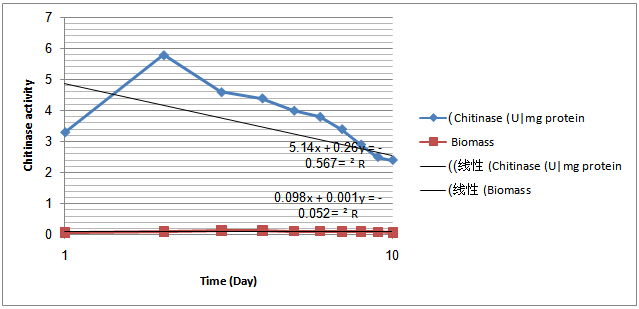
- Chitinolytic and antifungal activitiesThe endophyticS.hygroscopicuswas screened for chitinase production and inhibition of fungi some phytopathogensi.e. R.solani,F.oxysporum, A. alternate, A. niger, A.flavus, S. scleotiorumandB.cinerea.S.hygroscopicusproduced a high concentration of chitinase and inhibited the phytopathogens. S.hygroscopicusgenerally induced larger fungal growth inhibition zones for R. solani, S. scleotiorum,B.cinereaand F. oxysporum than for A.alternate ,Aspergillusand P.parasitica(Fig. 1).The production of extracellular chitinase enzyme by a potent antagonistThe production of extracellular chitinolytic enzyme in the strain was determined at different growth phases. The level of chitinase was sharply increased during the exponential phase and dramatically declined when the cells entered the stationary phase (Fig.2). The strain produced relatively high levels of chitinase (5.8 U/mg protein) at day1 of the incubation period.Purification of the chitinase
 | Figure 1. Chitinolytic and antagonistic activities of Sterptomyceshygroscopicus |
 | Figure 2. Time-course experiments related to growth, chitinase activity in the Sterptomyceshygroscopicusstrain |
 | Figure 3. Activity gels of extracellular lytic chitinase enzyme secreted by Sterptomyceshygroscopicusshowing detection of lytic enzyme activities on Native gel; M = Marker; Streptomyces =Strep |
 | Figure 4. Effects of temperature on the chitinaseactivityofStreptomyces shygroscopicus. |
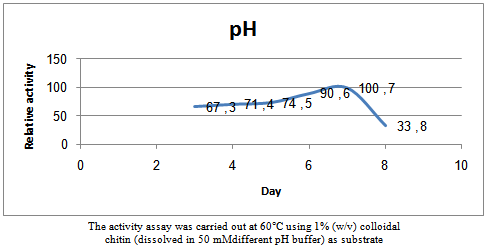 | Figure 5. Effects of pH on the chitinaseactivity ofSterptomyceshygroscopicus |
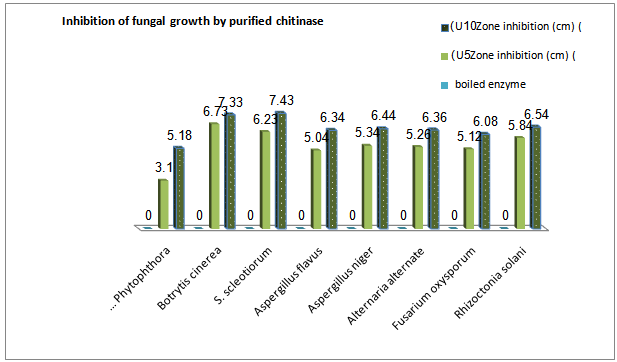 | Figure 6. Antifungal activity of chitinase fromSterptomyceshygroscopicusagainst somephytopathogens |
- Inhibition of fungal growth by purified chitinaseThe inhibition of the fungi phytopathogens i.e. R.solani,F.oxysporum, A. alternate, A.niger, A.flavus, S. scleotiorumand B.cinerea growth was observed on agar plates with paper discs that were coated with the purifiedchitinaseenzyme (Fig. 6). Antifungal activity of the purified chitinase of Streptomyces was confirmed. The inhibition diameter of the 10 U enzyme was greater than that of the 5 U enzyme, and the boiled enzyme had no inhibitory activity against all pathogens.
4. Discussion
- Microbial production of chitinase had great attention of both industrial and scientific environments, not only because of its wide spectrum of applications but also for the lacuna of an effective production method .Microorganisms, which secret a complex of mycolytic enzymes are considered to be possible biological control agents of plant diseases. Endophytes are micro-organisms that inhabit plant interior tissues. These microorganisms cause no harm to the host and do not develop external structures, which exclude nodulating bacteria and mycorrhizal fungi as endophytic micro-organisms[15]. Chitinolyticactivity has been implicated in the biocontrol activity of several bacteria, including Streptomyces spp. Owing to the innocuous nature of these organisms, deliverysystems to introduce endophytic bacteria, such asStreptomyces spp., into plants have been developed, andthe potential for endophytes as biocontrol agents has beenexplored[16]. In a recent study, anendophyticactinomycetes suppressed fungal pathogens, including R.solani, F.oxysporum, A. alternate, A.niger, A.flavus, S. scleotiorum and B.cinerea, indicating their potential use as biocontrol agents[1]. Shekharet al.[1]purified a bioactive compound from endophytic Streptomyces violaceusnigr that showed a strong antagonism towards various wood-rotting fungi, and chitinase enzymes wereassociated with this inhibition. In general, the higherchitinase activity was correlated with higher fungalinhibition. In the present study, the chitinolyticstrain, S.hygroscopicus showed high inhibition levels against fungi, and the fungal hyphae exhibited a degraded appearance after chitinolytic strain culture treatment. In this study on chitinase production, extra cellular chitinase activity was determined.The purification steps included ammonium sulfate precipitation, with columns of DEAE-Sepharose anion exchange chromatography and Sephacryl S-400 high resolution gel chromatography on AKTA purifier 100 protein liquid chromatography. The method gave a 5.4 fold increase of the specific activity and had a yield of 18%.Enzyme production was increased inpotential phase and more amounts weredetected in the stationary growth phase.Chitinases arefairly stable over broad pH range.Chitinase was optimally active at pH of 6.0 to 7.0 and at 30oC. The molecular weight of the chitinase was found to be around 80.8, 78 and 76 kDa by sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) technique. The chitanase were found to inhibit the growth of all phytopathogenic fungi i.e. R.solani,F.oxysporum, A. alternate, A.niger, A.flavus, S. scleotiorumand B.cinerea ,respectively.Since the cell walls of fungi consistlargely of chitin[17], it was postulated that chitinase produced by the antagonists could beinvolved in disease control. The production of theseenzymes was therefore used as the criteria forselection of potential biocontrol agents against pathogens.Streptomycesstrains are a likely choice as potential biological controlagents. Antagonistic activity of several Streptomyces sp.against a number of fungal pathogenic species has beenknown for a long time[18 , 19 & 20]. Also, ingreenhouse experiments, Streptomyces sp. has conferredvarious degrees of protection on different plant speciesagainst soil-borne pathogenic fungi[2].Very high protein content of clamydospores ofFusariumsp. (7 - 28%) may be responsible for their ability to resist lysis in soil. Studies on chitinolytic microorganisms have yielded a large increase in knowledge regarding their role in inhibition of growth of fungal plant pathogens (20 & 21). Still this knowledge is not sufficient enough to formulate a preparation based on these agents that can work efficiently in all different environmental conditions. Thebiocontrol agents are also affected due to the geological and environmental conditions. Studying the environmental conditions of the area where biological control agent has been employed and statistical based formulation approach techniques will increase life span of microorganisms in different ecological conditions.The results of this study led us to conclude that chitinase produced by endophytic Streptomyces has the potential for control of plant pathogenic fungi. This study provides a quantitative assessment of Streptomyces chitinolytic and antifungal activities.
References
| [1] | Shekhar, N., Bhattacharya, D., Kumar, D. and Gupta, R.K.(2006). Biocontrol of wood-rotting fungi with Streptomyces violaceusniger XL2. Can J Microbiol 52, 805–808. |
| [2] | El-Tarabily, K.A. and Sivasithamparam, K. (2006) Non-streptomyceteactinomycetes as biocontrol agents of soil-borne fungal plant pathogens and as plant growth promoters. Soil Biol. Biochem 38, 1505–1520. |
| [3] | Coombs, J.T., Michelsen, P.P. and Franco, C.M.M. (2004) Evaluation of endophyticactinobacteria as antagonists of Gaeumannomycesgraminis var. tritici in wheat. Biol Control 29, 359–366. |
| [4] | Dahiya, N.,RupinderTewari, Gurinder Singh Hoondal. (2006). Biotechnological aspects of Chitinolytic enzymes: a review. Applied Microbiol Biotechnology, 71: 773-782 |
| [5] | Yong,T., Jin Hong, Long Zhangfu, Zhang Li, Ding Xiuqiong, Tao Ke, GeShaorong, Liu Shigui (2005). Purification and characterization of an extracellular chitinase produced by bacterium C4.Annals of Microbiology, 55 (3): 213-218. |
| [6] | Nawani and Kapadnis. (2005). Diversity of Chitinases and their Industrial Potential.Current Perspectives and Potential Applications: 719-740. |
| [7] | Kuster, E. and Williams,S.(1964).Selection of media for isolation of Streptomycetes. Nature, 202: 928-929. |
| [8] | Domsch KW, Gams W, Anderson TH (1980). Compendium of soil fungi.Vol. 1, Academic Press, London, p. 589. |
| [9] | Shirling EB, Gottlieb D. (1966). Methods for characterization ofStreptomycesspecies.Int J SystBacteriol. 16:313–40. |
| [10] | Imoto T. and Yagishita K. (1971) .A simple activity measurement of lysozyme.Agri. Biol Chem. 35:1154–6. |
| [11] | Wang SL, Yieh TC and Shih IL. (1999). Production of antifungal compounds byPseudomonas aeruginosaK-187 using shrimp and crab shell powder as a carbon source.Enzyme Microb Technol. 25:142–8. |
| [12] | Laemmli, U. K. (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680-685. |
| [13] | Trudel, J. and A. Asselin. (1989). detection of chitinase activity after polyacrylamide gel electrophoresis. Anal.Biochem.178: 362_366 |
| [14] | Bradford, M. M. (1976) .A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal.Biochem.72, 248-254. |
| [15] | Azevedo, J.L. and Arau´ jo, W.L. (2007).Diversity and applications of endophytic fungi isolated from tropical plants. In Fungi: Multifaceted Microbes ed. Ganguli, B.N. and |
| [16] | Bhattacharya, D., Nagpure, A. and Gupta, R.K. (2007). Bacterial chitinases: properties and potential. Crit Rev Biotechnol 27, 21–28. |
| [17] | El-Tarabilya, K. A. ; Solimana, M. H. ; Nassara, A. H. ; Al-Hassania, H. A.; Sivasithamparam C,, K. ; McKennad , F. and Hardy, G. E. St. J. (2000). Biological control of Sclerotinia minor using a chitinolytic bacterium and actinomycetes. Plant Pathology 49, 573±583 |
| [18] | Crawford DI, Lynch JM, Whipps JM, Ousley MA (1993). Isolation and characterization of actinomycetes antagonists of fungal root pathogen. Appl. Environ. Microbiol. 59: 3899-3905. |
| [19] | Anitha, A. and Rabeeth, M. (2010).“Degradation of fungal cell walls of phytopathogenic fungi by lyticenzyme of Streptomyces griseus”, Afr. J. Plant Sci., 4, 61-66. |
| [20] | Subramaniam1, S., Ravi1, V., Narayanan1, G.. (2012). Studies on Production of Enzyme Chitinase from Streptomyces sp. and its anti-fungal activity.Journal of Pharmacy Research 2012,5(3),1409-1413. |
| [21] | Khamna S, Yokota A, Peberdy JF, Lumyong S (2009). Antifungal activity of Streptomyces spp. isolated from rhizosphere of Thai medicinal plants.; Int. Integr. Biol., 6(3): 143-147. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML