-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Microbiology Research
p-ISSN: 2166-5885 e-ISSN: 2166-5931
2012; 2(5): 127-132
doi: 10.5923/j.microbiology.20120205.03
Protease Production Capabilities of Micrococcus Luteus and Bacillus Species Isolated from Abattoir Environment
1Microbiology Department, University of Port Harcourt, Choba, Port Harcourt, Rivers State, Nigeria
2Microbiology Department, Federal University of Technology, PMB 1526, Owerri, Imo State, Nigeria
Correspondence to: Akujobi C. O. , Microbiology Department, Federal University of Technology, PMB 1526, Owerri, Imo State, Nigeria.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
The study investigated the optimum conditions of temperature, pH, inoculum size and time of incubation on bacterial protease production. Protease producing bacterial species were isolated from abattoir soil and identified as Micrococcus luteus and Bacillus species. The optimum conditions observed for protease production was 37℃ at pH 7, with 1% inoculum in the medium for 24 h of incubation in Micrococcus luteus while in Bacillus species, the optimum conditions observed was 47℃ at pH 9, with 2% inoculum concentration in the medium for 96 h of incubation. Generally, temperature and pH had more effect on the protease activity of Micrococcus luteus while inoculum concentration and time of incubation had more effect on the protease activity of Bacillus species. The study gave evidence that these bacterial isolates could be potentially applied in biotechnological processes.
Keywords: Protease Activity, Micrococcus Luteus, Bacillus Species, pH, Temperature, Inoculum Concentration, Time of Incubation
Article Outline
1. Introduction
- Microbial protease represents about 60% of all the industrial enzyme’s sales in the world due to their applications in several industrial sectors[1]. The induction of protease requires a substrate having peptide bonds including substrates like peptone, casein and other protein sources. The ammonia, as final product of enzymatic reaction of substrate hydrolysis, represses enzyme synthesis by a well-known mechanism of catabolite repression. This extracellular protease has also been commercially exploited to assist protein degradation in various industrial processes[2]. The great advantages offered by microbial enzymes are low material costs coupled with high and faster productivity and the ease with which the enzymes can be modified[3]. At present, due to high cost of substrates and mediums used, the overall cost of enzyme production is very high and therefore, development of novel processes to increase the yield of proteases with respect to their industrial requirements coupled with lowering down the production cost is highly appreciable from the commercial point of view[4].Proteases are complex multi-enzyme system which catalyzes the hydrolysis of amide bond in a protein molecular hence it has been used in the field of textile processing for degumming of silk and processing of wool[5-6]. With the advent of new frontiers in biotechnology, the spectrum of protease application has expanded into many new fields, such as clinical, medicinal and analytical chemistry. To meet the current largely increased demand, studies on the cost-effective production of industrially important enzymes have become the need of today.Microorganisms are the most important sources for enzyme production. Selection of the right organism plays a key role in high yield of desirable enzymes. For production of enzymes for industrial use, isolation and characterization of new promising strains using cheap carbon and nitrogen source is a continuous process. Habitats that contain protein are the best sources to isolate proteolytic microorganisms. Waste products of meat, poultry and fish processing industries can supply a large amount of protein rich materials for bioconversion to recoverable products[7].Proteases are present in all living organisms but microbial proteases are most exploited group of industrial enzymes. Based on their mode of action, they are further classified into four categories: alkaline, acid, thiol and metallo proteases. Alkaline (serine) Proteases are active over a broad pH (7-12) and temperature (35℃-80℃) ranges,[8], they are worldwide center of attraction for researchers. Several fungi, actinomycetes and bacteria are endowed with the capacity to produce alkaline serine proteases in diverse environmental and agro-climatic conditions. However bacterial proteases are preferred as they grow rapidly, need less space, can be easily maintained and are accessible for genetic manipulations. The important protease producing bacteria are species of Bacillus, Pseudomonas, Halomonas, Arthrobacter and Serratia. Among all bacterial specie, Bacilli play an important role in production of alkaline protease owing to their chemoorganotrophic nature, several species of Bacillus are industrially employed to produce thermostable alkaline protease as they grow easily under extreme pH and temperature conditions. The enhancement of protease production by genetic manipulation has been well studied in Bacillus cereus, Bacillus subtilis, Bacillus stearothermophilus etc, by a number of researchers, which further underlines the significance of this enzyme[8].The present study is aimed at isolation of protease producing bacterial species from abattoir environment and to determine the optimum conditions for protease activity.
2. Material and Methods
2.1. Sample Collection and Isolation of Proteolytic Bacteria
- The soil samples were collected from an abattoir environment in Owerri, Imo State, Nigeria. They were stored in ice and analyzed within one hour of collection. One gram of soil sample in a 250 ml flask was homogenized with 10 ml of sterile water; it was later made up to 100 ml with sterile water, mixed and shaken on a mechanical shaker for 45 minutes. An aliquot of the homogenized sample (0.2 ml) was spread on casein agar plates (nutrient agar supplemented with 36% casein) and incubated for 48 hours at 37℃. The isolates were identified based on their morphological and biochemical characteristics[9](Holt et al., 1994).
2.2. Screening of Proteolytic Bacteria
- The screening method described by Amara et al.[10](2009) was adopted. Briefly, to 100 ml of sterile water contained in a 250 ml flask was added 3 g of skim milk and autoclaved for 15 min. after sterilization, the suspension was decanted and the soluble solution was added to sterile water agar (16 g agar/l). The mixture was gently stirred until completely homogenized and then distributed in petri dishes. After media solidification, the bacterial cultures were inoculated on the plated and incubated at 50℃ for Bacillus species and 37℃ for Micrococcus luteus. Clear zones around the bacterial colonies indicate the presence of proteolytic activity which can be confirmed using coomassie blue staining method.
2.3. Coomassie Staining Method
- This was performed using the method of Weber and Osborn[11] (1969). Coomassie blue was dissolved in a solution of methanol-acetic acid-water (5:1:4[v/v/v]) to achieve 0.25% (w/v). Ten milliliter of the staining solution was added to each of the plates and incubated at room temperature for 15 min. After the incubation the staining solution was removed from the plate and the plate gently washed with distilled water. Thereafter, the plates were de-stained with a solution containing 66 ml methanol, 20 ml acetic acid and 114 ml of distilled water till there was a clear contrast between the plate’s background and the degradation zones around the bacterial colonies.
2.4. Protease Activity Assay
- The protease activity was determined according to the method of Anson[12](1938) with some modifications. The isolates were grown in a medium containing 10 g of glucose, 5 g of casein, 5 g of yeast extract, 2 g of KH2 PO4 and 10 g of Na2CO3 in 100 ml of sterile water. After sterilization, the medium was inoculated at 37℃ for 48 h. After incubation the culture filtrates were collected by centrifugation at 1000 x g for 12 minutes at 4℃. The supernatant was used as crude enzyme. 0.5 ml of the crude extract was mixed with 5.0ml of Tris-Hcl buffered casein and incubated at 37℃ for 30minutes. After incubation, 5ml of 110 mM trichloroacetic acid (TCA) was added to stop the reaction. The mixture was centrifuged at 10,000 rpm for 5 minutes and the released amino acids were measured as tyrosine using the method of Folin and Ciocalteu[13](1929) by calculating the amount of tyrosine in the extract using a tyrosine standard curve. The enzyme activity was expressed in units (U). One unit of enzyme was defined as the amount of enzyme that releases 1 μmol of tyrosine per mm of crude extract per minute.
2.5. Effect of Temperature on Protease Production
- The effect of temperature on protease production was studied by incubating the culture media at different temperatures ranging from 27℃-77℃ for 24hrs. Protease activity was determined after 24hrs of incubation.
2.6. Effect of pH on Protease Production
- The effect pH on protease production was determined by culturing the bacterium in the protease production media with different pH ranges (pH5 to 11). The enzyme assay was carried out after 24rs of incubation at 37℃.
2.7. Effect of Inoculum Concentration on Protease Production
- Effect of Inoculum concentration on protease production was determined by inoculating the production medium with different concentrations (2-7%) of overnight grown bacterial culture. The inoculated medium was incubated at 37℃ for 24hrs after which the culture medium was centrifuged at 5000 rpm at 4℃ for 15mins. The protease activity was determined as stated above. Statistical AnalysisData obtained from this study were analyzed using a two-way analysis of variance (ANOVA) and values for P≤0.05 were considered statistically significant.
3. Result
- Temperature had effect on the production of protease in both organisms. There was an increase in protease production with increase in temperature up to the temperature of 37℃ in Micrococcus luteus. After this temperature, the protease production started reducing gradually with increase in temperature till the end the work with the lowest protease production obtained at the temperature of 77℃, (fig. 1). The same observation was made in Bacillus species but the maximum protease production was obtained at the temperature of 47℃ (1.249 U/ml/min). Temperature had more effect on the protease activity of Micrococcus luteus than on Bacillus species although there is no significant difference on the temperature activities of the two organisms (P≤0.05). The result showed that both organisms were affected by increase in pH. In both organisms, protease production increased with increase in pH, (fig. 2). Their maximum protease productions were at pH 7 and 9, respectively for Micrococcus luteus and Bacillus species (1.23586 U/ml/min and 1.19804 U/ml/min, respectively). The lowest protease activities in both organisms were at pH 11. Statistical analysis showed no significant difference on the effect of pH on the protease activities of both organisms, (P≤0.05).
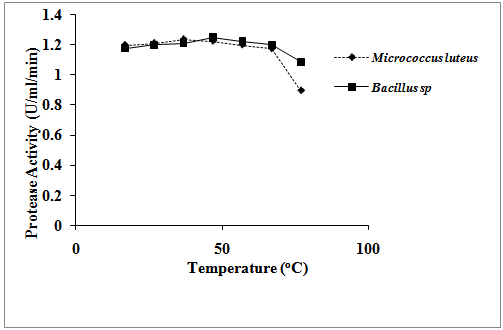 | Figure 1. Effect of temperature on protease activities of the isolates |
 | Figure 2. Effect of pH on protease activities of the isolates |
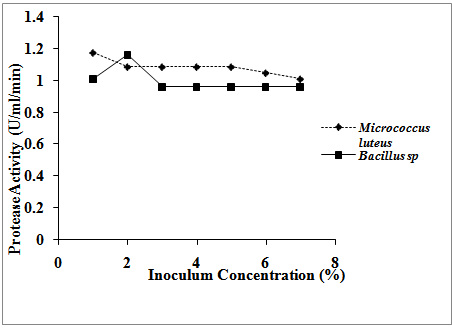 | Figure 3. Effect of inoculum concentration on protease activities of the isolates |
 | Figure 4. Effect of incubation time on protease activities of the isolates |
4. Discussion
- The study investigated the optimum conditions for protease production in Micrococcus luteus and Bacillus species. These parameters investigated included the effect of temperature, pH, inoculum concentration and time of incubation on the production of protease. It was discovered that the parameters investigated had varying effects on the protease activities of the isolates. On temperature effect, it was discovered that there was an increase in protease production with increase in temperature up to the maximum protease activities at the temperature of 37℃ and 47℃ for Micrococcus luteus and Bacillus species, respectively. Kalaiarasi and Sunitha[14] also reported a similar trend in Pseudomosa fluorescens where they observed that the organism could produce protease in the range of 27-57℃ with production maximum at 37℃. However, increase in temperature beyond 37℃ and 47℃ for Micrococcus luteus and Bacillus species, respectively led to decline in protease production proving that temperature plays a major role in enzyme production.The pH of the culture strongly affects many enzymatic processes and transport of compounds across the cell membrane. The protease activities of the isolates were also affected by the pH of the medium. Increase in pH resulted in corresponding increase in protease production up to the maximum protease production at pH 7 and 9, respectively for Micrococcus luteus and Bacillus species (1.23586 U/ml/min and 1.19804 U/ml/min, respectively). The result showed that there was a stimulation of the enzyme production at neutral pH for Micrococcus luteus and alkaline pH for Bacillus species. The result obtained is in consonance with the work of Kumar et al.[15] who reported the protease production was at maximum at pH 7 and 9 for Bacillus sp. and Pseudomonas sp. respectively although vary in the organisms involved. This same result was also obtained by Amara et al.[10] and Sathees Kumar et al.[16] who observed an optimum protease activity of 34 Unit/ml and 215.56 U/ml for Geobacillus sp and Pseudomonas aeruginosa, respectively at pH 9 and the work of Sally[17] who, using Azocoll as a protease substrate, observed highest specific protease activity of Burkholderia strain 2.2 N at pH 7.5. Production of protease at alkaline pH has been reported by so many authors. The bacterial isolate, Bacillus amovivorus, was found by Sharmin et al.[18] to exhibit maximum protease production at medium pH 8.5 but the highest biomass yield was recorded at medium pH 7.0. Aftab et al.[19] also reported that alkaline protease production by Bacillus brevis was found in alkaline medium at pH 10.5 Previously, Freshteh et al.[20] also reported production of alkaline protease by Bacillus strain L2 at pH 10.5. Maximum protease production from the genus Bacillus was observed by Mahendran et al.[21] at pH 8.0 while at pH 10, the protease production was about 60%.Initial inoculum concentration influenced the production of protease in both organisms. It was observed that increase in inoculum concentration increased the protease activity in both organisms up to the maximum of 1.17282 U/ml/min at 1% inoculum concentration for Micrococcus luteus and 1.16021 U/ml/min at 2% Bacillus species. Increasing the inoculum concentration beyond these percentages resulted in decrease in the protease activity in both organisms. These results were partly in accordance with Elibol et al.[22] who reported that 2.5% inoculum level gives higher protease production.Time of incubation also had effect on the protease production of the two bacterial isolates. The result of the effect of time of incubation on protease production showed that there was a drastic decrease in protease production with increase in the time of incubation in both organisms. The highest protease activity observed in both organisms was at 24 and 48 hours of incubation in both organisms. This finding is in partial agreement with the work of Kumar et al.[15] who reported that Pseudomonas sp. S22 showed a peak for protease production at 24 h of incubation and again at 108 h of incubation. However, the present result is in total agreement with the work of Kalaiarasi and Sunitha[14] who observed the same peak protease production at 24 h of incubation.
5. Conclusions
- The data gathered in this study has provided evidence for the protease producing ability of abattoir-soil-isolated Micrococcus luteus and Bacillus species. The influence of environmental factors on the protease production of the isolates was also evident in this study. This study has given a hint that the microbial wealth of protease producing bacteria isolated from abattoir environment can be harnessed for biotechnological processes. The appreciable high enzyme activity at alkaline pH suggested that Bacillus species is a potential producer of alkaline proteases which can find application in detergent and textile industries.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML