-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Microbiology Research
p-ISSN: 2166-5885 e-ISSN: 2166-5931
2012; 2(5): 118-122
doi: 10.5923/j.microbiology.20120205.01
Interference of Mycoplasma spp. or Ureaplasma spp. in Ovine Semen Quality
Lilian Gregory 1, Huber Rizzo 1, Natália C. Gaeta 1, Gabriela Tortorelli 1, Maristela V. Cardoso 2, Elena Mettifogo 3, Melissa Buzinhani 3, Jorge Timenetsky 3
1Department of Medical Clinic, Faculty of Veterinary Medicine, University of São Paulo, 05508 270, Postcode, Brazil
2Laboratory of Bacterial Reproduction, Center for Animal Health, Biological Institute of São Paulo, São Paulo, 04010-970, Brazil
3Mycoplasma Laboratory, Institute of Biological Sciences, University of São Paulo, São Paulo, 05508-900, Brazil
Correspondence to: Lilian Gregory , Department of Medical Clinic, Faculty of Veterinary Medicine, University of São Paulo, 05508 270, Postcode, Brazil.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
The presence of mycoplasma in ovine semen was associated in 9.09% (3/33) to the microscopic and macroscopic alterations of this fluid. Mycoplasma spp. was isolated in 36.36% (12/33) from semen samples while Ureaplasma spp. was isolated in 12.12% (4/33). The mollicute infection rates in studied semen samples indicated that the diagnosis of these bacteria in ovine must be a routine procedure for the quality of this biological product.
Keywords: Mycoplasma,Ureaplasma, Ovine Semen
Article Outline
1. Introduction
- The reproductive disorders in animals interfere the economic investments in cattle breeding. The related diseases may be caused by handling deficiencies, environmental and genetic influences and infectious agents. The urogenital infections may also be caused by some Mollicutes and Mycoplasma spp. and Ureaplasma diversum are mostly detected or isolated and associated with the reproductive disorders in bovines[1,2].In 1955 Albertsen, in Denmark raised the possibility that the antibiotic-resistant microorganisms were responsible for the persistent low fertility bulls[25]. The first mycoplasma was isolated in 1898 by Nocard and Roux in France in a clinical material obtained from a bovine during an outbreak of contagious bovine pleuropneumonia.A bovine Ureaplasma was isolated firstly in 1978 and by the time many bovine reproductive disorders were controversially associated because this mollicute was also isolated from healthy animals. This bacteria was studied through experimental and natural infections in bovines causing abortion, infertility and vulvovaginitis syndrome[3,4,5,6]. Ball and Mc Caughey[7] mentioned that the presence of mycoplasmas in vulvar or cervix side may be normal but their isolation from cervix or uterus was considered a pathological condition.The association of Mollicutes with reproductive disorders in caprines and ovines was mentioned less frequently [8-10]. Mycoplasma serotype 11-strain 2D, is a not yet classified strain and was related with cases of vulvovaginitis and reproductive disorders in Australia, USA, India, England, France and Nigeria[10]. Other species were isolated from reproductive tract of ovine and caprine with circunstantial evidences of pathogenesis. In this context are included M. mycoides subsp. mycoides, M. bovigenitalium, M. agalactiae, M. mycoides subps. capri, M. arginini, M. alkalesecens e Acholeplasma spp.[10,11,12]. Fe et al.,[13] obtained three positive PCRs and cultures for Mycoplasma spp. in 146 samples of caprine semen. Using serological and PCR methodology the isolates were identified M. agalactiae . Livingston et al.,[14] and Ball et al.,[7] inoculated in caprines and ovines field isolates of ureaplasma recovered from other diseased animals and related them to the serotype IX as causative of infertility and abortion in sheep.Actually seven species of ureaplasma are recognized. They are human, bovine, canine, feline and avian origin. It was described other five species, but they also did not received yet a nomenclature[15]. These isolates were included in those recovered from ovine or caprine and nine described serotypes. Their DNA and some polypeptides pointed a similarity with U. diversum[8,16,17]. The pathogenicity of these microorganisms in reproductive disorders in caprines and ovine are unknown. The adherence of mentioned Mollicutes to the bovine spermatozoa is the initial step in this host-parasite relationship. Nicholas et al. (1999) described decreased motility of spermatozoa in semen of bulls belonging to herds infected with Mycoplasma ovine/caprine serogroup 1. These animals presented low fertility rates, and degeneration of the tail in cases of orchitis. AK et al (1995) inoculated experimentally M. agalactiae and observed a decrease of volume, activity, motility and semen concentration, with an increase in abnormal sperm. Some species of Mycoplasma and Ureaplasma diversum were associated with seminal vasculitis, balanoposthitis, epididymitis,[27] other functional disorders and morphology of spermatozoa[28,29] including a reduced motility.[30,31,[32,33,34,35,36]The virulence mechanisms by which organisms Family Mycoplasmataceae cause problems for cells are related to interaction with the immunological system, changes in humoral and cellular immune response and the production and induction of cytotoxic components such as ammonia and peroxidase.[38] Mycoplasma spp and Ureaplasma diversum has a worldwide distribution and can be spread through international trade of animals, semen and industrial products for transferring embryos.[30,37,38]In this view it was compared the presence of mycoplasma in ovine semen and the influence of this infection in macroscopic and microscopic aspects of this fluid.
2. Material and methods
2.1. Microorganisms
- Ureaplasma diversum ATCC49783, U. urealyticum T-960, Mycoplasma capricolum, M. conjuntivae HRC581, M. arginini G230, Acholeplasma laidlawii PG-8.
2.2. Sampling
- Ovine semen was obtained by electro-ejaculation from 33 ovine (breed Santa Inês, Poll Dorset e Texel ). The animals were from three properties of a region named Piedade/SP-Brasil. The clinical samples were aliquoted in vials for culturing, microscopic and macroscopic analysis.
2.3. Mycoplasma culturing and characterization
- The ATCC strains of mycoplasma and ureaplasma were inoculated respectively in SP4 and UB agar. The UB was with manganese sulphate. The UB broth was adapted from U9C because was added with de CMRL-1066 as in SP4. Semen samples were diluted previously in SP4 or UB broths to 10-3 and inoculated in respective media. The incubation was at 37ºC. The inoculated UB broth were in aerobiosis and the SP4 broth were at 10% of CO2. The cultures were incubated for 21 days and observed for the production of small fried egg colonies for Mycoplasma spp or dark brown granulated colonies for Ureaplasma spp. The ureaplasmas were also characterized for alcalinization of UB broth due urea hydrolysis by urease activity[5,18].
2.4. Semen Analysis
- The semen samples were observed for physical, morphological and cellular aspects. The used parameters were the mentioned by Colégio Brasileiro de Reprodução Animal[19].
2.5. Statistical analysis
- Risk estimative, Odds Ratio, was obtained by software GrapfPad Instat, version 3. for Windows.
3. Results
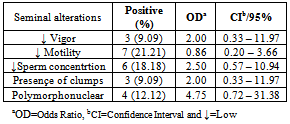
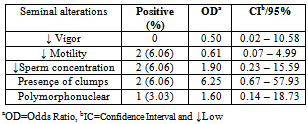
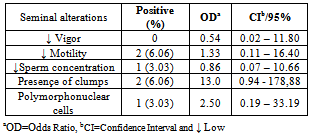
- The reference strains grew in SP4 and UB media. Mycoplasma spp. was isolated in 12 (36.36%) semen samples while Ureaplasma spp. was isolated in four (12,12%) samples. It was observed an association of Mycoplasma spp e Ureaplasma spp. in 9.09% (3/33) with semen alterations in some cases. All positive cultures to mycoplasma were not associated with semen alterations. The Mycoplasma spp. isolates produced “fried egg” colonies and was not retained by 0.45μm porosity membrane. The Ureaplasma spp. isolates also produced typical colonies and increased the pH of UB broths. Four semen samples were contaminated and did not allow the mycoplasma isolation. Tables 1, 2 and 3, presents the results from cultures and risk rates for the alteration of semen aspects.
|
|
|
4. Discussion
- Mycoplasma isolation from ovine semen was not reported yet in Brazil. Although it was studied 33 samples the obtained frequencies of 36.36% for Mycoplasma spp. and 12.12% for Ureaplasma spp. are the first national reference and points that the percentage of this infection is probably higher. Isolation of mycoplasmas in ovine is not usually performed in other countries. Kappor et al.,[11], in India, Mycoplasma spp. and Acholeplasma spp. were isolated in 17.6% from 68 semen samples. Trichard et al.,[20], in South Africa found mycoplasmas in 83% of samples from ovine with ulcerative balanosposthitis and vulvovaginitis and 36% in healthy animals. Nicholas et al.,[10], in Great Britain reported the isolation of Mycoplasma serogroup 11 in vaginal mucus from 23 female infertile sheep. It was showed the interference of these microorganisms in ovine breeding.Contamination of semen and preputial mucus with U. diversum was firstly mentioned in 1969 in England, when ten preputial cultures and 84% in 32 samples of fresh semen were positive for the agent.[40] ONOVIRAN et al., (1975), in Canada observed 132(35%) positive cultures for U. diversum in preputial mucous, 140(24%) in fresh semen samples and 42(14%) in processed semen. In 1978, Czechoslovakia, this ureaplasma was recovered from 202 samples of bull semen in insemination centers.[41]The highest rate of positive cultures for Mycoplasma (71%) was obtained in cows with low fertility compared with 24% of cows culled for other reasons.[42]FISH et al. (1985), Canada, reported that 28% of bulls used for artificial insemination had semen contaminated with species of Mycoplasma and M. bovis was not recovered. GARCIA et al. (1986), in the same country, studied 2950 samples of semen and M. bovis was. Ball et al. (1987)[43] isolated Mycoplasma spp in 46% of 332 samples of fresh semen of bulls In 32% of these positive samples, were also infected with U.diversum.LE GRAND et al. (1995)[44], in France, isolated U. diversum in 74% (37/50) of semen samples. Serogroups B and C were predominant in males.M. bovis was the first bovine mycoplasma identified in Brazil by ROSSINI, (1978)[45] in calves with pneumonia in a property located in State of São Paulo. Subsequently, LIBERAL et al. (1982)[46] reported the isolation of Mycoplasma spp. in cases of bovine pneumonia in the State of Rio de Janeiro.The mycoplasma transmission through the infected semen justify in part the diversity of foundings. Lingwood et al.,[21] and Quinn et al.,[22] showed the adherence of U. diversum to bovine spermatozoa trough sulfoglicolipids. Eagleasome and Garcia[22] described the interference of M. bovis in the fertilization processes. The presented data and the diversity of the host-parasite relationship in mycoplasmology strongly suggest a better control of these bacteria in ovine breeding or rising.The motility and other parameters of ovine spermatozoa analysed in present study were influenced by mycoplasma infection. It was possible to estimate a risk of 4.75 higher to found these bacteria in polymorphonuclear-PMN cells in infected ovine semen (Table. 1). When the semen is infected with Ureaplasma spp the estimative risk to produce semen clots was 6,25 higher if compared with mycoplasma free semen. It was detected an OR>1 for a lactescent appearance, spermatozoa density and PMN (Table 2). The same OR and semen parameters was obtained for the co-infection of Mycoplasma spp and Ureaplasma spp. In this study the estimated risk was 1,33, 13, 2,5 times higher for alterations of spermatozoa motility, clots and PMN in semen respectivily(Table. 3). Cardoso et al.,[24], studied bovine semen infected with Mycoplasma spp and obtained a risk of 1.02 higher for the lactescent semen and 2.7 higher for spermatozoa density. In presence of U. diversum the risk was 1.32 higher for clumping. In the present study it was detected a higher risk in ovine semen quality when infected with mycoplasma than described for bovines. Nicholas et al.,[10] mentioned a decreasing motility of bovine spermatozoa and low fertility in reproductive cattle infected with Mycoplasma serogroup 11. Animals with orchitis presented a degeneration of spermatozoa tails. Ak et al,.[12], experimentally inoculated M. agalactiae in bovine semen and mentioned a decreasing volume, motility and density of spermatozoa and an increase of abnormal spermatozoa. In 1977 Jurmanova and Sterbova associated a low sperm motility(60%) and the semen contamination by mycoplasma and ureaplasma adhered on these cells . It was observed by a reduced motility (60%) and[26].There are infectious agents more commonly associated with animal reproductive disorders : Brucella spp, Campylobacter spp, Letospira spp.[28][35] However, many other are less mentioned, as Mollicutes, listed in schedule B of the OIE, and therefore are extremely important and should be studied.[39]Eaglesome (1980) studies found that M. bovis interfered extensively in various stages of fertilization in vitro, first causing the decrease in the percentage of sperm capacitation, fusion with the oocyte and interfering with the organization of the pronucleus after chromatin decondensation, without, however, cause changes in their viability and motility.
5. Conclusions
- It was possible to found estimate risk of 4.75 higher in polymorphonuclear-PMN cells in infected mycoplasma ovine semen and 6,25 higher estimate risk semen clots in semen infected with Ureaplasma spp. In this study the estimated risk was 1,33, 13, 2,5 times higher for alterations of spermatozoa motility, clots and PMN in infected semen respectivily. The monitoring of mycoplasma infection in ovine production must be included in routine analysis.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML