-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Microbiology Research
p-ISSN: 2166-5885 e-ISSN: 2166-5931
2012; 2(4): 114-117
doi: 10.5923/j.microbiology.20120204.09
Molecular Epidemiology of Genital Chlamydia Trachomatis Infection in Asymptomatic Adolescent-Young People
Cecilia Cuffini 1, 2, 3, Marina Bottiglieri 4, Ximena Kiguen 1, Carlos E. Alonso 1, Romina Valdes Deimundo 1, María Beatriz Isa 5, Roxana Cannistraci 6, Silvia Gonzalez 6, Alicia Farinati 5
1Institute of Virology, National University of Cordoba. Cordoba, 5016, Argentine
2Fleming 3498, Barrio: Lago Azul Villa Santa Cruz del Lago Có
3rdoba 5152. Argentine
4Laboratory of Microbiology, Reina Fabiola Clinic, Catholic University of Cordoba, Cordoba, 5000 Argentine
5University del Salvador. Buenos Aires, C1023AAC, Argentine
6Department of Microbiology, School of Medicine, National University of Cordoba. Cordoba, 5016, Argentine
Correspondence to: Cecilia Cuffini , Institute of Virology, National University of Cordoba. Cordoba, 5016, Argentine.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Infections by Chlamydia trachomatis are usually asymptomatic or minimally symptomatic and for this reason, diagnosis is often delayed. The aim of this study was to investigate the genotype of genital Chlamydia trachomatis infections among asymptomatic adolescent and young people. Detection and genotyping of Chlamydia trachomatis were performed with polymerase chain reaction (PCR) and restriction fragment length polymorphism analysis directly on crude cells of first-void urine. Crude samples (n=427; 222 men/205 women) from young people with different social and economic conditions were screened for C. trachomatis. The prevalence of chlamydial infection was 13.7% among women and 4.1% among men. Socioeconomically disadvantaged women had a significantly higher prevalence compared with the rest of the women 27.4% vs. 6.1% (p=0.0006). Genotype E was the most frequently found (N=27 73%) among both genders. Screening programs focussed on sexually active adolescents and young people attending health clinics are important for detection of Chlamydia trachomatis infections.
Keywords: Chlamydia Trachomatis, Asymptomatic, Young People
1. Introduction
- Infections by Chlamydia trachomatis are the mostprevalent sexually transmitted bacterial infections recognized throughout the world. The World Health Organization[1] estimated 92 million new cases worldwide in 1999 and indicates that the incidence of the infection has continued to rise every year in both industrialized and developing countries.The original Wang and Grayston classification[2] that defined . trachomatis serovars was based on antigenic differences of the major outer membrane protein (momp). According to the reclassification of the order Chlamydiales of 1999, the family Chlamydiaceae is now divided in two genera, Chlamydia and Chlamydophila[3]. The genus Chlamydia comprises the species C. trachomatis, C. suis and C. muridarum.C. trachomatis is the causative agent of a variety of diseases and syndromes, including trachoma (genotypes A, B, Ba, C), urogenital infections, chlamydial conjunctivitis, infant pneumonia (genotypes D-K), and lymphogranuloma venereum (genotypes L1, L2, L2a, L3)[4,5].Lymphogranuloma venereum (LGV) is endemic in several areas of developing countries and represents a major risk factor for HIV acquisition[6,7].More than 70% of genital infections in women and 50% in men are asymptomatic[8,9]. The factors that determine whether infections are symptomatic or asymptomatic are still unknown. From the public health perspective, this leads to the persistence of undetected C. trachomatis reservoirs with significant perpetuation of transmission. Thus, an efficient and rapid method for detection and typing of C. trachomatis genotypes, particularly a highly sensitive method that allows the finding and analysis of low number of copies of C. trachomatis DNA is needed, mainly for the study of asymptomatic populations. Compared to other techniques, PCR provides higher sensitivity and specificity. Reports have shown that plasmid-targeted PCR is more sensitive than ompA gene-targeted PCR[10]; however the last one allows detection of strains that lack plasmids[11].Furthermore, PCR has been successfully applied to different Chlamydia genotypes by means of restriction fragment length polymorphism (RFLP) analysis and direct sequencing of amplified ompl DNA[12,13]. Genotypic characterization of C. trachomatis isolates can not only provide valuable insights into circulating C. trachomatis serovars within a given community, but can also improve understanding of their epidemiology, which may assist in developing strategies to increase the control of sexually transmitted disease (STD).The aim of this study was to genotype C. trachomatis samples recovered from non-symptomatic adolescent-young people with different socio economic conditions from several areas of city.
2. Material and Methods
- Urine samples from 427 individuals (205 females/222 males) were collected in two-year periods. The inclusion criteria were: sexually active asymptomatic individuals aged 18 to 24 years who had not received antimicrobial therapy during the 15 days prior to the study; females should have a negative pregnancy test. Written informed consent was obtained and the project was approved by the Ethics Council of the Catholic University of Córdoba. A survey was used to collect the data about socio economic conditions. The sample included 272 individuals with satisfied basic necessities (SBN) and 155 individuals under socio economically disadvantaged conditions (SDC). These conditions included precarious housing, parents with null or basic educational level, overcrowded accommodations (three or more persons per room) or lack of toilet.Data Processing: The data were processed and tabulated and the variables were quantified; the distribution and trend measures were determined and the analysis of independent variables was performed with statistics tests. Data were compared by univariate analysis with Pearson test and odds ratio.Univariate comparison between patients with Chlamydial infection and control group was performed using χ2 test. Variables with significance level lower than 0.1 on univariate analysis were analyzed with a multivariate logistic regression model using a forward conditional (likelihood ratio) method.Urine: Subjects were instructed not to urinate within the previous hour and females should not clean the perineum before urinating. The first catch of 10 to 20 ml of urine was collected in a clean collection cup and refrigerated immediately at 2-8℃ for no more than 4 days for PCR.Plasmid PCR and ompA PCR: In order to determine the sensitivity of plasmid PCR and ompl PCR, a dilution assay was performed with genotype L2/434/Bu. This assay showed that the plasmid PCR was capable to detect 0.1 inclusion-forming units. Ompl PCR was about 100 times less sensitive than plasmid PCR; for this reason, when making the Hemi-nested PCR for MOMP, sensitivity values were equalled.PCR pre-treatment methods: Two hundred micro litters of the samples were centrifuged at 16,000 x g for 30 min. The pellet was resuspended in 200 μl of Tris-HCl (pH 7.5), stored at -80℃ and thawed before use. This freezed-thawed suspension was used for PCR. The subsequent pre-treatment method was digestion with proteinase K (0.5 mg/ml) in the presence of non-ionic detergent Tween 20 (0.45%). The solution used for pre-treatment contained KC1, MgCl2, and Tris-HCl (pH 8.3). All pre-treated samples were boiled for 10 minutes before PCR.Detection of C. trachomatis by plasmid PCR: The primers used for generating a 201-bp fragment of the C. trachomatis cryptic plasmid were CTP1 (plus strand; 5'-TAGTAACTGCCAClTCATCA-3') and CTP2 (minus strand; 5'-TTCCCCTTGTAATTCGTTGC-3')[13].C. trachomatis ompA PCR and genotyping of C. trachomatis were performed as previously described[13]. Briefly, the primers used for generating an approximately 1.1-kb fragment of the ompl gene were SEROlA (plus strand; 5'-ATGAAAAAACTCTTGAAATCGG-3') and SERO2A (minus strand;5'-TTTCTAGAT/CTTCATT/CTTGTT-3').PCR products were analyzed by 1.5% agarose gel electrophoresis. Briefly, 2 µl of the primary ompA PCR product was pipetted with an aerosol-tipped pipette into a PCR mixture containing the SERO2A primer and one nested primer, pCTM3(5'-TCCTTGCAAGCTCTGCCTGTGGGGAATCCT-3') located 55 bp downstream of SEROlA. The amplification conditions of the nested PCR were the same as those of the primary ompA PCR.RFLP analysis of ompA PCR- and nested PCR-positive samples was carried out as described by Lan et al.[13] with minor modifications. Briefly, 10 μl of positive PCR product were digested with 2.5 U of AluI (Pharmacia, ) according to the manufacturer's instructions. If necessary, samples were analyzed with a second enzyme (Hinfl, CfoI, or a combination of EcoRI and DdeI). The samples were then analyzed by 3% agarose gel electrophoresis, stained with 10 μg of ethidium bromide per ml during 45 min and photographed under UV light. The patterns observed for each genotype were in accordance with those reported by Lan et al [13]. The samples were amplified with primers for β-globin in order to detect the inhibitor´s presence.
3. Results
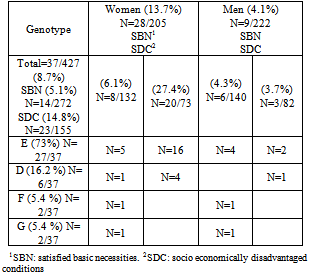
- Of the 427 individual screened, 8.7% (N=37) yielded positive results with plasmid PCR; 11 samples that were negative by ompA PCR were positive by plasmid PCR; all of these were clearly positive after Hemi nested ompA PCR. Plasmid free C.trachomatis strains not were detected in this study.The prevalence of C.trachomatis in the female groups was 13.7% (28/205) and 4.1% in males (9/222). The difference was statistically significant, p<0.0004, OR 3.74 (1.66-9.24, IC 95%).The prevalence was 5.1% (14/272) in the SBN group and 14.8% (23/155) in the SDC group. Socioeconomically disadvantaged women had a significantly higher prevalence compared with the rest of the women 27.4% vs. 6.1% (p=0.0006). The difference was statistically significant, p<0.0006, OR 3.21 (1.52-6.97, IC 95%).Genotyping of C. trachomatis in urine samples was carried out in 37 ompA PCR-positive samples. Genotyping of these samples showed that they were identified directly after AluI digestion.
|
4. Discussion
- The nucleic acid amplification tests worked significantly better than alternative methods like culture isolation or ELISA [14] for the detection of C. trachomatis both for urine samples and cervical swabs [15].The existence of a stable plasmid-free C. trachomatis may cause undesirable problems when using PCR for diagnosis, although it is 10 times more sensitive that ompA PCR. In our study, both PCRs demonstrated to have no differences regarding sensitivity, possibly due to the fact that nested ompA Hemi nested PCR was performed.Studies among clinically healthy populations have shown a prevalence rate equal or major than values detected in our population of asymptomatic young people (8.7%). Two reports have shown a lower prevalence rate of 0.9 % in the [16] and [17] in a population of military and adolescent students; however, higher prevalence rates (8.8%) have been reported for Chinese students[18] and young people of (8.3%)[19]. The rate for a population who attended health care centers in showed an average prevalence rate of 11.7%, ranging from 4 to 25.7%[20,21]Infection with C. trachomatis can be asymptomatic in up to 80% of the females[22], this fact can difficult its diagnosis and detection. The asymptomatic nature of chlamydial infection makes screening essential for the control of this infection. C. trachomatis has its highest prevalence among young people. More than 13.5% of the women younger than 25 years old present infection of the lower genital tract; however, this rate decreases to less than 4.9% in women over 25 years old[23].We found large variations of the incidence of C. trachomatis among different groups of subjects within Córdoba, a large city of . We correlated the dependent contribution of socio economic conditions to the risk of acquiring C. trachomatis infection. We detected extremely high rates of C. trachomatis infection among asymptomatic young women with socio-economical disadvantages. Genotype E was the most frequently found genotype among women of the SDC group.A high prevalence of genotype E (27 of 37, 73%) and the lack of associated clinical symptoms may suggest that this genotype is more successful than other less-prevalent genotypes in our studied population. Indeed, a successful genotype would be the one that remains undetected for longer periods of time, enhancing dissemination. Other studies have demonstrated frequencies of genotype E similar to ours in asymptomatic subjects [24-25]. Even among High-Risk Women in , serovar E was associated with asymptomatic infection [26]. In city, , Gallo Vaullet et al have confirmed an increased frequency of genotype E in neonatal ocular samples and genital adult samples [27].This technique has provided a valuable and sensitive resource for molecular epidemiological analysis to identify high-risk groups. To the best of our knowledge, this is the first study of genotype distribution of C. trachomatis in asymptomatic young people in Córdoba Argentina.In conclusion, we detected a high prevalence of C. trachomatis genital infection among healthy young people in , by using a highly sensitive diagnostic PCR.
ACKNOWLEDGMENTS
- The work was supported by the Alberto Roemmers Foundation, .
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML