-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Microbiology Research
p-ISSN: 2166-5885 e-ISSN: 2166-5931
2012; 2(3): 51-55
doi: 10.5923/j.microbiology.20120203.02
Improved Production of Gibberellic Acid by Fusarium moniliforme
Vidhya Rangaswamy
Industrial Biotechnology Group, Reliance Life Sciences Pvt. Ltd.
Correspondence to: Vidhya Rangaswamy , Industrial Biotechnology Group, Reliance Life Sciences Pvt. Ltd..
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Optimization studies for improvement in the yield of gibberellic acid production by Fusarium moniliforme in submerged and solid-state fermentation is the focus of this paper. In the current study, use of jatropha seed cake as substrate for solid-state fermentation resulted in an unprecedented gibberellic acid yield of 105 mg/g of moldy bran. A 2.5 fold increased in the titre resulting in 15 g gibberellic acid /L could also be obtained by optimization of physiological parameters in submerged fermentation. This is the first study reporting such high yield of gibberellic acid and presenting a commercially viable production process using cheap substrates.
Keywords: Process Optimization, Submerged Fermentation, Solid-State Fermentation
Article Outline
1. Introduction
- Gibberellic acid (GA3), the most important gibberellin, is a class of diterpenoid that functions as plant growth regulator [1]. It affects stem elongation, elimination of dormancy, flowering, sex expression, enzyme induction and leaf and fruit senescence. GA3 is a high valued industrially important biochemical with various applications in agriculture with price ranging around $ 25/g in the international market[1-3]. GA3 is presently produced largely by submerged fermentation techniques using Fusarium moniliforme or Gibberella fujikuroi[4]. Other bacteria that belong to the genus Azotobacter and Azospirillum[5] also synthesize GA3. Recently, a Pseudomonas sp. isolated from wastes of processed olive has also been shown to produce GA3 (285 mg/L)[6]. The factors that account for high cost of GA3 in present market scenario are the low yield of GA3 produced and its presence in dilute form in submerged fermentation; leading to higher costs of downstream processing and disposal of waste water. GA3 can also be produced by the solid-state fermentation (SSF), which has got a tremendous potential for production of secondary metabolites. There are many advantages that make the SSF process commercially viable such as greater yields, lower energy consumption, a lesser environmental impact of the process, and differential expression of metabolites. The yields obtained from SSF are decent enough to offset the higher costs of downstream processing, thereby lowering the cost of gibberellic acid.In the present study, optimization of production of GA3 by SSF and submerged fermentation, using Fusarium moniliforme has been investigated. An economically viable process for commercial production of GA3 is described.
2. Materials and Methods
2.1. Organism and Growth Conditions
- Fusarium moniliforme NCIM 1100 was obtained from National Collection of Industrial Microorganisms, Pune, India. The strain was cultured and maintained on Potato dextrose agar (PDA) slants.
2.2. Submerged Fermentation
- F. moniliforme culture was inoculated from the PDA slants into 250 ml of CD broth (composed of (g/L) sucrose, 30; NaNO3, 3; K2HPO4,1; MgSO4.7H2O, 0.5 ; KCl, 0.5 and FeSO4, 0.01, pH 6.0) and incubated at 30C for 10 days at 150 rpm. Cell growth was monitored every 24 h and GA3 was estimated in the supernatant. All optimization experiments including effect of varying pH (5, 7 and 8) and temperature (23, 25, 30 and 37℃) were carried out in CD broth. Effect of carbon source was evaluated by replacing sucrose with either glucose, galactose, xylose, glacial acetic acid or methanol at a final concentration of 20 g/L in CD broth. All experiments were carried out at least in triplicates to ensure reproducibility.
2.3. Solid Substrate Fermentation
- For preparation of inoculum, the fungus was grown in 100-ml Erlenmeyer flasks containing 25 ml CD broth at 150 rpm at 30℃ for 4 days. Jatropha seed cake was obtained after extraction of oil from Jatropha curcas seeds. For SSF using jatropha seed cake as substrate, 5 g of the cake was mixed with 8 ml of mineral salt solution (CuSO4, 0.007g; FeCl3, 0.007g; and ZnSO4, 0.007g dissolved in 1 liter of 0.2 mol/L HCl). The initial moisture content of the medium was adjusted to 60%. The sterile production medium was inoculated with 3.5 ml of 4-day old inoculum of F. moniliforme, mixed thoroughly and incubated at 30℃ for 10 days at 45o angle. The production of GA3 was monitored every 2 days up to 10 days.
2.4. Analytical procedures
- GA3 was estimated spectrophotometrically by the method described by Berriso et al[7] at 254 nm. GA3 was also detected by HPLC method at 206 nm on a C18 column using methanol: water (3:1) as the mobile phase at 1 ml/min flow rate[8]. The GA3 elutes in 3 min under these conditions. Qualitative determination of GA3 was done by TLC as described by Puchooa et al[9]. The GA3 extracted from the fermentation was dissolved in ethanol and separated by TLC using isopropanol – ammonia - water (10:1:1, v/v/v) as mobile phase. The plates were sprayed with 3 % (v/v) H2SO4 in methanol containing 50 mg FeCl3 and heated in oven at 800C for 10 min. GAs fluoresce and appear as greenish spot under UV light.
2.5. Extraction of GA3 from the SSF
- Gibberellins were extracted from SSF by adding 100ml of distilled water to moldy bran in each flask. The mixture was kept on shaking incubator at 150 rpm for 2 h. The slurry from each flask was filtered through muslin cloth and the volume of the filtrate was made to 100 ml. Filtrate was centrifuged at 10,000 rpm for 10 min at 28℃. Supernatant was collected and analyzed for GA3 concentration spectrophotometrically. All experiments were performed in triplicate.
2.6. Purification of GA3 from the SSF extract
- Isolation of GA3 from the SSF extract was done by the method described by Ergun et al[10]. Briefly, to 5 ml of extract, 60 ml of solvent consisting of methanol, chloroform, and 2 N ammonium hydroxide (12:5:3 v/v) and 25 ml of distilled water was added. The mixture was shaken well in a separating funnel. After removal of the bottom chloroform layer, the methanol in the upper aqueous layer was evaporated. The pH of the remaining solution was adjusted to 2.5 and extracted thrice with 15 ml of ethyl acetate per cycle. The ethyl acetate phase was collected and evaporated to dryness. The dried material was dissolved in 5 ml of ethanol and GA3 was estimated.
3. Results and Discussion
- The present study is aimed at improvising the production of the agriculturally important growth hormone, GA3, using submerged and solid-state fermentation strategies.
3.1. Submerged fermentation in Czapek – Dox broth
- The growth and GA3 production of F. moniliforme culture in Czapek – Dox (CD) broth was monitored. After an initial lag of 2 days, there was an exponential increase in growth (data not shown). The log phase continued up to 4 days before reaching the stationary phase. Production of GA3 started from 6th day and peaked on the 8th day reaching a concentration of about 5.8 g/L. The production remained constant thereafter.
3.2. Physiological optimization
- It is known that physiological factors considerably influence the GA3 production in submerged fermentation[3,6]. To improve the yield of GA3 in the production medium, growth parameters including pH, temperature, incubation time and media were optimized.
3.2.1. Optimization of pH in Submerged Fermentation
- Effect of initial pH of the medium on GA3 production was investigated (Figure. 1A). It was noted that initial pH of the medium did not greatly influence the production of GA3 although highest yield of 6.5 g/L was obtained on the 8th day when the initial pH was adjusted to 7.0. Similar profile was reported for GA3 production in Pseudomonas wherein a maximum yield of 0.3 g/L was obtained at pH 7.0[6]. However, Borrow et al[11] reported that GA3 production decreases when the pH was outside the range of 3.0-5.5 in a stirred culture. The growth of the fungi was however better at pH 8.0 (data not shown).
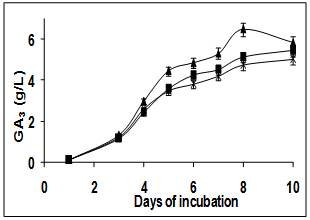 | Figure 1A. Effect of pH on GA3 production in submerged fermentation at pH 5 (-■-), pH 7 (-▲-) and pH 8 (-X-) |
3.2.2. Optimization of Temperature in Submerged Fermentation
- Role of temperature on growth of F. moniliforme and production of GA3 was evaluated. Incubation at 30℃ was optimum for GA3 as the yield increased to 5.8 g/L which corroborates well with the production profile in published reports[1,6] (Figure. 1B). The growth was however better at 23 and 25℃ as compared to 30℃ indicating a distinct difference in conditions for growth and production of GA3.
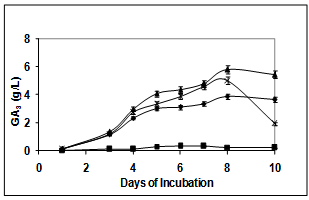 | Figure 1B. Effect of temperature on GA3 production by F. moniliforme in submerged fermentation at 23℃ (-X-), 25℃ (-♦-), 30℃ (-▲-) and 37℃ (-■-) |
3.2.3. Optimization of Carbon Source in Submerged Fermentation
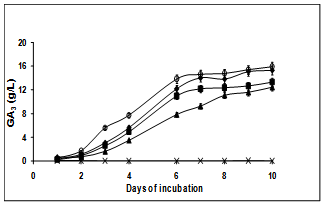
- To determine the role of individual carbon source favoring high yields of GA3 production, sucrose in the CD medium was replaced with glucose, galactose, xylose, glacial acetic acid or methanol at a final concentration of 20 g/L. Of all the carbon sources, sucrose was the best giving a yield of 15 g/L under optimized conditions. Glucose was found to be equally effective whereas all other carbon sources gave lower or no GA3 production (Figure. 2) in 10 days. This is by far the highest yield reported through submerged fermentation.
3.3. Solid-State Fermentation
- Kumar and Lonsane[12] have comprehensively reviewed the potential of the SSF technique for GA3 production and have carried out various investigations. GA3 fermentation in the present study was carried out by solid-state fermentation using jatropha seed cake as a substrate. Jatropha seed cake is a readily available waste product from biodiesel plant wherein oil extracted from seeds of Jatropha curcas is transesterified into biodiesel. Jatropha seed cake is a relatively recalcitrant lignocellulosic substrate having a cellulose content of 15 % and lignin content of 30 %.In the present report, jatropha seed cake was used as a substrate for SSF. Interestingly, an unprecedented yield of 105 mg GA3/g of substrate was obtained by 4th day and remained constant thereafter (Figure. 3). This is so far the best reported yield of GA3 obtained by SSF. This is the first report where the feasibility of using jatropha seed cake as a substrate for SSF has been investigated. The 5-fold improvement in the yield in our studies compared to that reported in the literature is unarguably contributed by the substrate jatropha seed cake. The seed cake may be providing the right combination of carbon and nitrogen to the fungus for production of GA3. Several fungi are known to be cellulolytic in nature and most commonly used cellulases that have been used in biomass pretreatment process are from Trichoderma reesei and Aspergillus niger[17]. The GA3 producer, F. moniliforme is also known to be cellulolytic in nature[18]. This attribute of the fungi may be enabling it to utilize the cellulosic sugars from the substrate more effectively thereby resulting in high yield of GA3. Detailed analysis of the factor(s) responsible for promoting such high yields of GA3 is warranted. The jatropha seed cake is a waste from the biomass industry is toxic due to the presence of phorbol esters and may need detoxification prior to being used as land feed or animal feed. However, they can be used ‘as is’ as substrate for production of this valuable phytohormone.
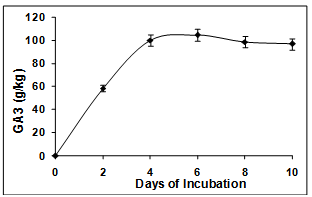 | Figure 3. Solid-state fermentation for production of GA3 using jatropha seed cake as substrate |
4. Conclusions
- A process for production of very high titres of gibberellic acid production by solid-state fermentation is described. The process is easily scalable and employs cheap raw materials rendering it economical.
Acknowledgements
- The authors gratefully acknowledge the encouragement and support of Reliance Life Sciences Pvt. Ltd., in carrying out the research work.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML