-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Microbiology Research
2012; 2(2): 26-35
doi: 10.5923/j.microbiology.20120202.05
Effectiveness of Rhizosphere Bacteria for Control of Root Rot Disease and Improving Plant Growth of Wheat (Triticum aestivum L)
Seema Dua , S. S. Sindhu
Department of Microbiology, CCS Haryana Agricultural University, Hisar-125004, India
Correspondence to: S. S. Sindhu , Department of Microbiology, CCS Haryana Agricultural University, Hisar-125004, India.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Pathogenic fungus Rhizoctonia solani causes root rot disease in wheat leading to collapsing of the aerial part of the plant. To characterize antagonistic bacteria, one hundred and thirty bacterial isolates were obtained from the rhizosphere soil of wheat and these rhizobacterial isolates alongwith 72 reference strains were screened for their antagonistic interactions against R. solani under cultural conditions. Sixteen bacterial isolates inhibited the growth of R. solani and growth inhibition zone varied from 6- by different rhizobacterial isolates. Two isolates WPS3 and WPS90 caused maximum growth inhibition of the fungi. Growth inhibiton of the pathogenic fungi was also observed using culture filterates of antagonistic rhizobacterial isolates. The protein estimation of the culture filterates showed that the amount of protein excreted by different rhizobacterial isolates varied from 3.6 to 33.0 mg ml-1 of the supernatant. The loss of antagonistic activity after treatment with proteinase K and high temperature treatment indicated that excreted proteins are responsible for the antagonism. Pot house studies showed that inoculation of R. solani in wheat caused 85-90% root rot disease incidence at 60 to 90 days of plant growth. The single inoculation of rhizobacterial isolate WPS3 resulted in 131% increase of plant dry weight as compared to uninoculated control plants. The coinoculation of isolate WPS3 with R. solani enhanced 115% plant dry weight whereas coinoculation of Pseudomonas isolate WPS90 caused 98% increase in plant dry weight in comparison to control uninoculated plants at 90 days of plant growth. Coinoculation of Pseudomonas isolates WPS3 and WPS90 with R. solani also caused 88.9 and 66.7% disease control, respectively at 90 days of plant growth. Thus, Pseudomonas isolate WPS3 could be further exploited for plant growth improvement under field conditions.
Keywords: Rhizosphere bacteria, Rhizoctonia solani, Biological control, Pseudomonas sp., Bacillus sp., Root rot disease, plant dry weight
Article Outline
1. Introduction
- The plant rhizosphere is an important ecological environment in soil for plant-microbe interactions. These interactions with plants could be beneficial, neutral or with detrimental effects resulting in plant diseases[1-3]. The pathogenic microorganisms cause various plant diseases that usually weaken or destroy plant tissues and reduce crop yields varying from 25% to 100%. Root diseases are estimated to cause 10-15% yield losses annually in the world. These plant diseases are mostly controlled by application of chemical pesticides. However, the widespread use of chemical pesticides has been a subject of public concern due to potential harmful effects on the environment, their undesirable effect on non-target organisms and the possible carcinogenicity effect of some chemicals. Moreover, the patho- gens develop resistance against the pesticides applied. Therefore, biological control offers an alternative approach to the use of costly and harmful chemicals, and provides low cost, environmental friendly control measures to reduce the activity of plant pathogens[4-5]. Antagonistic rhizosphere microorganisms inhibit the growth of pathogenic microorganisms without disrupting the ecological balance and thus, biological control strategies are highly compatible with the sustainable agriculture.The saprophytic, pathogenic and plant growth promoting strains of bacteria have been found to colonize the plant rhizosphere[6]. Field application of some rhizosphere bacteria has resulted in significant promotion of root biomass, plant growth and yield of different crops[2, 7-9]. These beneficial bacteria are generally referred as plant growth promoting rhizobacteria (PGPR)[10]. The beneficial effects of PGPR have been correlated with increased recycling, solubilization and uptake of mineral nutrients[11], synthesis of vitamins, amino acids, auxins and gibberellins[12, 13], and by antagonism of potential plant pathogens[14-16]. The antagonistic microorganisms by their interactions with various soil-borne plant pathogens play a major role in biological disease control[5, 17]. Therefore, Bacillus and Pseudomonas species that can establish in rhizosphere are ideal candidates for use as inoculant to enhance plant growth and as biocontrol agents for suppression of plant diseases under pot house and field conditions[7, 18].Wheat is the second most important grain crop and is a source of staple food in many countries of the world. Though the production of wheat has increased after green revolution but the attack of various diseases like head blight, powdery mildew, root rots, rusts, smuts, take-all and Karnal bunt of wheat has greatly affected its yield and quality[19-21]. Root rot disease in wheat is caused by fungus Rhizoctonia solani that produces reddish brown lesions on the root surface just below the soil line. It causes hindrance to absorption of water and minerals through the roots leading to collapsing of the aerial part of the plant. In this study, bacterial isolates obtained from wheat rhizosphere were tested for growth inhibition of pathogenic fungi R. solani on medium plates and the antagonistic Pseudomonas sp. were inoculated onto wheat for plant growth improvement and control of root rot disease under pot house conditions.
2. Materials and Methods
2.1. Isolation of Bacterial Cultures from the Rhizosphere Soil
- One hundred and thirty rhizobacterial isolates were obtained from the rhizosphere soil of wheat by serial dilution plate method using King’s B medium. Soil samples were collected randomly from the rhizosphere of wheat at 60 and 90 days of plant growth from 3 different locations of CCS Haryana Agricultural University, Hisar farm. From each location, samples were collected from six different sites. The serial dilutions of the soil samples were made up to 10-5 and 0.1 ml of diluted soil suspension was plated on King’s B (KB) medium plates[22]. The plates were incubated at 28+ in BOD incubator for 3-4 days. Pseudomonas, Bacillus and other rhizobacterial colonies were selected based on typical morphological and pigment production characteristics. Seventy two reference strains were procured from the Department of Microbiology, CCS Haryana Agricultural University, Hisar. The rhizobacterial strains/isolates were maintained by periodic transfer on Luria Bertani agar slants[23]. Rhizobacterial isolates showing zone of inhibition were screened for oxidase test, catalase test, spore staining and Gram staining[24]. These bacterial cultures were stored at in refrigerator for further use.
2.2. Host Species
- Seeds of wheat (Triticum aestivum L.) variety WH542 were obtained from Department of Seed Science and Technology, CCS Haryana Agricultural University, Hisar.
2.3. Screening of Rhizobacterial Isolates for Antagonistic Activity Using Fungal Pathogens
- The interaction of rhizobacterial isolates with Rhizoctonia solani was studied by the spot test method on PDA medium plates[25]. The fungi R. solani was grown on PDA slants and spore suspension of fungi was prepared in 3 ml sterilized water. Two ml of fungal spore suspension (containing 5.2-6.0 x 108 spores ml-1) were incorporated into molten PDA medium and plates were prepared. Growth suspension of 48 h old rhizobacterial isolate (2.0 µl) was spotted on preseeded plates. Plates were incubated for 48 h at 28+ and growth inhibition of fungi R. solani was recorded. Rhizobacterial isolates showing zone of inhibition were selected.
2.4. Determination of location of Antimicrobial Substance
- Bacterial isolates were grown in LB medium broth for 2, 5 and 10 days at 28+2°C in the incubator with a rotary shaker (100 rpm speed). Bacterial growth suspension (1.0 ml) was centrifuged at 10,000 rpm for 20 minutes to separate cells and cell free culture filterate (supernatant). Seven mm diameter wells were made into the PDA agar medium plates with the help of sterile cork borer, preseeded with spore suspension of the fungi. Cell free culture filtrate (50 µl) obtained from selected bacterial isolates was loaded in the wells and plates were incubated at 28+2°C. Observations for antifungal activity against R. solani were scored by measuring growth inhibition zone on PDA medium plates after 2 days.
2.5. Determination of Nature of Antimicrobial Substance
- To determine nature of antimicrobial substance, the culture supernatants were analysed for production of specific proteins. Culture filterates of rhizobacterial isolates were precipitated with concentrated trichloro-acetate solution (100%). The precipitated proteins were solubilized in phosphate buffer saline (PBS) (pH 7.0) and used for study of growth inhibition against R. solani by spot test method on PDA medium plates. Protein concentrations in the total exoproteins were determined by the method of Lowry et al.[26]. using bovine serum albumin (BSA) as standard. Absorbance at 690 nm (A690) of different samples wells was measured in a spectrophotometer. A standard curve of BSA concentration (µg ml-1) versus A690 was drawn and the regression equation was obtained using MS Excel programme and the protein concentration in the test samples was determined.Protein obtained from culture supernatants of rhizobacterial isolates were treated with proteinase K enzyme. Stock solution of proteinase K was prepared by dissolving 3.0 mg of proteinase K into 600 µl Tris-EDTA buffer. Protein samples mixed in Laemmli buffer were treated with proteinase K (50 µg ml-1) and the mixture was incubated for 90 minutes at . The suspension was incubated for 10 min at and after cooling Proteinase K was again added to a final concentration of 100 µg ml-1. The preparation was then incubated for another 60 minutes at . Samples were also treated with high temperature by incubating for 45 minutes at . Samples treated with proteinase K and high temperature were tested for antagonistic activity.
2.6. Inoculation with Pseudomonas Strains for Control of Root Rot Disease in Wheat
- Two selected Pseudomonas isolates i.e., WPS3 or WPS90 were tested for disease control and plant growth promotion of wheat under pot house conditions. Six treatments were made and each treatment had three replications as described below. (i). T1: Soil (control, uninoculated)(ii). T2: Soil + Pseudomonas isolate WPS3(iii). T3: Soil + Pseudomonas isolate WPS90(iv). T4: Soil + R. solani (v). T5: Coinoculation of R. solani + WPS3(vi). T6 : Coinoculation of R. solani + WPS90Sandy loam soil was collected from CCS Haryana Agricultural University farm dry-land area. The earthen pots of capacity were filled with sandy loam soil and washed river sand, mixed in 70:30 ratio. The cultures of Pseudomonas isolates were grown on LB medium slants for 2 days. About 3 ml of sterilized water was added to Pseudomonas cultures. The bacterial growth was scrapped and vertexed on rotary shaker to get uniform suspension. The growth suspension of Pseudomonas culture WPS3 (5.2 × 108 cells ml-1) was inoculated on the roots of wheat plants after germination in the T2 and T5 treatments only and isolate WPS90 (5.7 × 108 cells ml-1) was inoculated in the T3 and T6 treatments.The seeds of wheat (Triticum aestivum L.) variety WH542 were surface sterilized with acidic alcohol (70:30) for three minutes and 4-5 washings were given with sterilized water. Surface sterilized seeds were inoculated with broth culture of Pseudomonas isolates. The viable count in the broth was kept 108-109 cells ml-1 and seeds were inoculated with 2 ml of bacterial growth suspension[27]. Growth of R. solani (4 days old) was harvested from PDA plates with the help of inoculation needle and then sterilized saline water was added to get uniform fungal growth suspension. Fungal growth suspension (50 ml) was mixed in soil: sand mixture in earthern pots in treatments T4, T5 and T6. The growth suspension of fungus was also inoculated on the roots of wheat plants after germination in the R. solani treatments only as control treatment. The plants were grown in the pot house under day light conditions during November 2008 - March 2009. The plants were uprooted at 60, 75 and 90 days of growth and observations were taken for the dry weight of root and shoot and control of plant disease.
2.6.1. Plant Fresh and Dry Weight
- Shoot and root portions of the plants after uprooting were weighed first. Then they were dried in oven at for 24h and weighed again.
2.6.2. Disease Index and Reduction in Disease
- On the basis of symptoms observed percent disease index, percent final stand and percent disease control were calculated by the formulae.
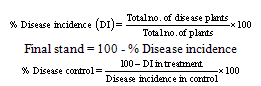 Disease control and disease incidence were recorded at 60, 75 and 90 days of sowing. It was calculated on the average of five plants grown per pot.
Disease control and disease incidence were recorded at 60, 75 and 90 days of sowing. It was calculated on the average of five plants grown per pot.3. Results
- Certain bacteria isolated from rhizosphere soils possess properties that allow them to exert beneficial effects on plants either by enhancing crop nutrition or by reducing damages caused by pathogens or pests. Some of these rhizosphere bacteria, such as Pseudomonas and Bacillus have emerged as important biological inputs of agricultural soils. During the present investigation, the effect of bacterial cultures on the inhibition of growth of fungal pathogen Rhizoctonia solani (causal agent of root rot of wheat) was tested under culture conditions. The control of root rot disease of wheat was examined using rhizobacterial isolates under pot house conditions.
3.1. Isolation of Rhizobacteria from the Rhizosphere Soil
- Bacterial isolates were obtained from soil samples collected from rhizosphere of wheat by dilution plate method using King’s B medium. Both fluorescent and non-fluorescent Pseudomonas and Bacillus isolates were obtained. Originally, one hundred thirty bacterial colonies were selected based on morphological and pigment production characteristics. For oxidase test, different rhizobacterial isolates were grown on KB medium plates for 2 days at 28+. One percent solution of tetramethyl-p- phenyl-diamine dihydrochloride was added to cover surface of plates. Seventy four isolates were found oxidase positive and these isolates belong to Pseudomonas species. Fifty six isolates showed Gram positive staining reactions, catalase positive and formed spores indicating that these isolates belong to Bacillus species.
3.2. Screening of Bacterial Cultures for Growth Inhibition of Fungi
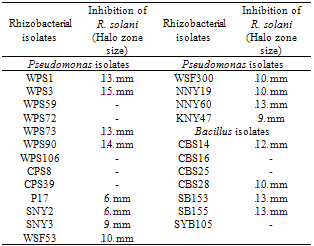
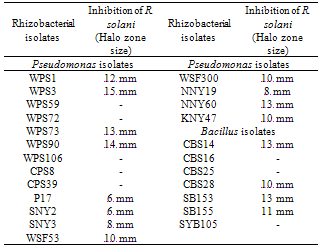
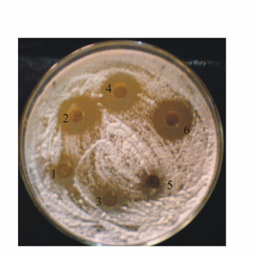
- All the 202 rhizobacterial isolates/strains were screened for their antagonistic interaction against the fungi i.e., R. solani on PDA medium plates using spot test method[25]. Detection of antagonistic activity for rhizobacterial isolates depended on the ability of bacteria to inhibit fungal growth under cultural conditions. Out of 202 rhizobacterial isolates tested, 16 strains were found to inhibit the growth of R. solani (Table 1; Fig. 1). The fungal growth inhibition zone varied from 6-15 mm with different strains/isolates tested. Eight rhizobacterial isolates i.e., WPS1, WPS3, WPS73, NNY60, CBS14, WPS90, SB153 and SB155 showed 12-15 mm growth inhibition zone. Rhizobacterial isolate WPS3 showed maximum inhibition zone () whereas, isolate WPS90 showed inhibition zone against R. solani under cultural conditions. Out of 16 antagonistic isolates, 12 rhizobacterial isolates belonged to Pseudomonas sp. and 4 cultures were found Bacillus sp.
|
3.3. Effect of Cell Free Culture Filtrate on Growth Inhibition of R. solani
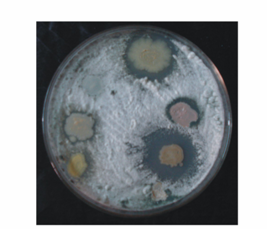
- Culture filtrate of selected rhizobacterial isolates were tested for inhibition against fungal pathogens by spot test method. The cell free culture filtrate obtained from all the antagonistic isolates, inhibited the fungal growth of R. solani and the zone of inhibition varied from 6-15 mm (Table 2; Fig. 2). Cell free culture filtrate of WPS3 and WPS90 caused maximum inhibition of the fungi. More inhibition of fungi was observed with culture filtrate obtained from 10 day-old growth of bacterial culture. Rhizobacterial isolate WPS3 showed inhibition zone against R. solani, whereas isolate WPS90 showed inhibition zone. The cell free culture filtrates obtained from bacterial culture NNY19 showed maximum inhibitory activity at 5th day but did not inhibit fungal growth at 10th day of growth. Thus, cell free culture filtrate studies of the antagonistic cultures showed that antagonistic substance is extracellular.
|
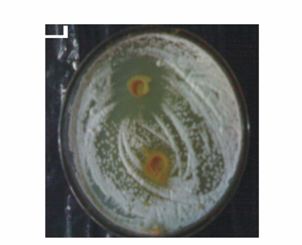 | Figure 2. Antagonistic activity of cultural filterates obtained from rhizobacterial isolates WPS 3 and WPS 106 (control) against R. solani seeded on PDA medium plates |
3.4. Nature of Antimicrobial Substance
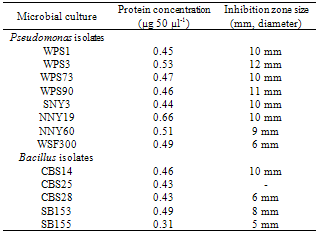
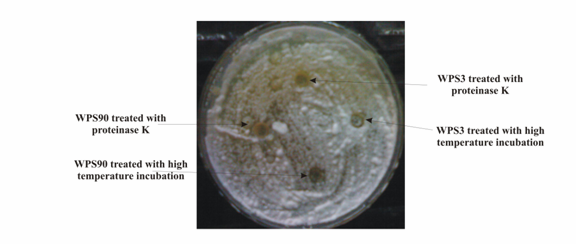
- To determine the nature of antimicrobial substance, culture filtrate of bacterial isolates were analysed for production of specific proteins. Culture filtrate of selected rhizobacterial isolates i.e., WPS1, WPS3, WPS73, WPS90, NNY19, NNY60, WSF300, SNY3, CBS14, CBS25, CBS28, SB153 and SB155 were treated with concentrated trichloroacetate (TCA) and resultant protein precipitates were solubilized in PBS buffer. Proteins (50 µl) were loaded in wells made in PDA medium plates that were preseeded with spore suspension of R. solani. The concentration of loaded proteins varied from 0.31-0.66 µg 50 µl-1 of sample. The fungal growth inhibition zone varied from 6- after 48 h of incubation (Table 3; Fig. 3). Maximum inhibition zone was shown by proteins obtained from Pseudomonas cultures WPS3 () and WPS90 (). Presence of inhibition zone by loading of precipitated proteins on PDA medium plates showed that the antimicrobial substance could be of proteinaceous nature.
|
3.5. Effect of Inoculation of Pseudomonas and R. Solani on Root Rot Disease Control and Plant Growth of Wheat
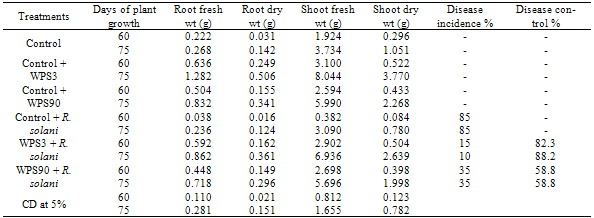
- Wheat seeds inoculated either singly with Pseudomonas isolate WPS3 or WPS90 and/or with R. solani fungi were grown in pots containing soil and sand mixture. Fungal growth suspension (50 ml) was mixed in the pot containing soil. Inoculated plants were grown under day light conditions in the pot house during the month of November 2008 to February 2009. Single inoculation with Pseudomonas strain WPS3 resulted in 76.3% increase of plant dry weight at 60 days of plant growth in comparison to uninoculated control plants. Inoculation of R. solani caused root rot disease in 85.0% of the inoculated plants (Table 4). Coinoculation of WPS3 with R. solani enhanced 70.2% plant dry weight as compared to uninoculated control plants. Single inoculation with WPS90 resulted in 46.3% increase of plant dry weight, whereas its coinoculation with R. solani enhanced 34.4% plant dry weight as compared to uninoculated control plants. The coinoculation of Pseudomonas strain WPS90 with R. solani showed 58.8% disease reduction whereas 82.3% disease reduction was observed with Pseudomonas strain WPS3.At 75 days of plant growth, single inoculation with Pseudomonas isolate WPS3 caused 256% increase in root dry weight and 249% increase in shoot dry weight (Table 4) whereas inoculation with isolate WPS90 resulted in 140% increase in root dry weight and only 116% increase in shoot dry weight as compared to control uninoculated plants. Coinoculation of isolates WPS3 and WPS90 with the fungi showed 238% and 156% gain in shoot dry weight, respectively. Maximum 88.2% disease control was observed on coinoculation of Pseudomonas isolate WPS3 with R. solani and only 58.8% disease reduction was observed on coinoculation of Pseudomonas isolate WPS90.
|
|
4. Discussion
- Microbial populations having pathogenic, saprophytic and plant-growth promoting ability colonize the same ecological niche rhizosphere[6] and interact with each other as well as with the plant through symbiotic, associative, neutralist or antagonistic effects[1, 28, 29]. Several rhizosphere bacteria have been reported with the potential to control various root and foliage diseases of agricultural crops[15, 30-32].One hundred and thirty bacterial isolates were obtained from the wheat rhizosphere based on morphological and pigment production characteristics. Seventy four isolates belonged to Pseudomonas species whereas fifty six Gram positive isolates belonged to Bacillus species. Gupta et al.[33] isolated rhizobacteria from the rhizotic zones of green gram using 7 selective and 4 non-selective media. Gram negative bacteria accounted for 65% out of 121 bacteria isolated and dominant genera were Pseudomonas, Bacillus, Enterobacter, Proteus and Klebsiella. Similarly, Pseudomonas was found most predominant (42%) followed by Bacillus (28%) and Enterobacter (21%) in rhizosphere and rhizoplane of groundnut[34].Screening of rhizobacterial isolates for fungal growth inhibition on PDA plates showed that only 7.92% cultures possess the ability to inhibit pathogenic fungi R. solani in cultural conditions (Table 1). Results of oxidase test, catalase test, Gram and spore staining showed that 12 antagonistic rhizobacterial isolates belonged to Pseudomonas sp. and 4 isolates were found Bacillus. Growth inhibition of the fungi by different rhizobacterial isolates varied from 6- (Table 1; Fig. 1). Pseudomonas isolate WPS3 showed maximum inhibition zone () followed by isolate WPS90 that showed inhibition zone against R. solani. Khot et al.[35] isolated 36 rhizobacteria from rhizosphere of chickpea and five bacteria were found to inhibit the growth of Fusarium oxysporum and Rhizoctonia bataticola. Siddiqui et al.[8] showed that Pseudomonas aeruginosa and Bacillus subtilis strains produced inhibition zones by inhibiting the radial growth of Macrophomina phaseolina, Fusarium oxysporium and Rhizoctonia solani. Lemessa and Zeller[36] found that six strains of rhizobacteria i.e., RP87, B2G, APF1, APF2, APF3 and APF4 showed good inhibitory activity against Rhizoctonia solanacearum out of 118 strains tested. reported that siderophore production and antifungal activity was exhibited by 10 to 12.77% of Azotobacter and Pseudomonas isolates. Pseudomonas Ps5 and Bacillus B1 isolates showed broad-spectrum antifungal activity on Muller-Hinton medium against Aspergillus, Fusarium and Rhizoctonia bataticola. Similarly, Karuppiah and Rajaram[16] showed that eight Bacillus sp. out of 63 different Bacillus isolates exhibited plant growth promoting activities and six of these Bacillus isolates also inhibited the growth of Penicillium sp., Cercospora sp. and Fusarium oxysporum.The cell free culture filtrate obtained from antagonistic rhizobacterial isolates showed inhibition of R. solani growth on medium plates and inhibition zone varied from 6- (Table 2; Fig 2). Cell free culture filtrate obtained from WPS3 and WPS90 caused maximum inhibition of the fungi. Inhibitory effect observed with culture filterate of the antagonistic rhizobacterial isolates indicated that antifungal compound is of extracellular nature. Maximum fungal growth inhibition zone was obtained from the supernatants of 10 day-old growth cultures suggesting that the secondary metabolites may be produced in more quantity at this growth phase of the antagonistic culture. Various studies have shown that secondary metabolites produced by rhizobacterial cultures are involved in antagonism of fungal pathogens[37, 38]. Nagarajkumar et al.[39] found that oxalic acid (OA) detoxifying fluorescent P. fluorescens strain PfMDU2 was most effective in inhibiting the mycelial growth of R. solani in vitro. Several proteins were detected in the culture filtrate of P. fluorescens strain PfMDU2 when it was grown in medium containing oxalic acid. The plasmid-deficient strain (PfMDU2P−) failed to grow in medium containing OA and did not inhibit the growth of R. solani. Similarly, fluorescent Pseudomonas isolates PGC1 and PGC2 were found to produce chitinase and β-1, 3-glucanase that inhibited the growth of R. solani and Phytophthora capsici[40]. Chitinase and β-1, 3-glucanase were involved in the inhibition of R. solani, whereas antifungal metabolites of non-enzymatic nature were found responsible for inhibition of P. capsici.Extracellular proteins secreted by antagonistic cultures were found to inhibit the fungal growth on PDA medium plates that was preseeded with R. solani and growth inhibition zone varied from 6- (Table 3; Fig. 3). Maximum inhibition zone was observed from the proteins obtained from Pseudomonas cultures WPS3 () and WPS90 (). The results suggested that excreted proteins obtained from culture filtrates of antagonistic bacteria are responsible for the inhibition of fungi under cultural conditions. The loss of antagonistic activity after treatment with proteinase K and high temperature incubation indicated that antifungal compound was of proteinaceous nature. Similar high temperature treatment given to broth culture of Lysobacter enzymogenes strain 3, inactivated the cells and lytic enzymes did not show inhibition zone against F. graminearum under in vitro conditions[41]. The SDS-PAGE analysis of total proteins obtained from different selected antagonistic isolates followed by Coomassie blue staining showed that four protein/polypeptide bands, i.e., 22, 25, 45 and 86 KDa are common in all the antagonistic isolates (data not shown). It showed that any or all of the four protein/polypeptides could be responsible for antagonism of R. solani. Grover et al.[42] reported that antifungal compound produced by Bacillus subtilis RP24 was proteinaceous in nature. Partially purified methanol fractions on SDS-PAGE showed one protein/peptide band with molecular weight between 1.0-1.5 kDa whereas no band was observed for the negative mutant establishing the proteinaceous nature of the compound. The extracellular, methanol soluble, thermostable and pH-stable antifungal metabolites were characterized as cyclic lipopeptides belonging to the iturin group of peptide antibiotics. Disease suppressive pseudomonads were found to produce an antifungal polyketide (2, 3-deepoxy-2, 3-didehy- drorhizoxin)[43]. A significant relationship between the antagonistic potential of P. fluoresecens strain MDU2 against Rhizoctonia solani and its production level of extracellular β-1, 3-glucanase, chitinase, salicyclic acid and hydrogen cyanide was observed[44]. They also reported that extracellular chitinase and laminarinase produced by P. stutzeri had marked effect on mycelial growth inhibition rather than spore germination. Inoculation of wheat with Pseudomonas isolates WPS3 or WPS90 resulted in significant increase in plant dry weight as compared to control uninoculated plants at all the three stages of plant growth (Table 4, 5). Maximum increase of plant dry weight (135.8%) was observed with single inoculation of Pseudomonas strain WPS3 in comparison to 46.6% increase in plant dry weight due to single inoculation of WPS90. Inoculation with R. solani caused root rot disease in 85% of inoculated plants. However, the coinoculation of Pseudomonas isolate WPS3 with R. solani lowered the disease incidence and 88.2% disease reduction was observed (Table 4). Whereas, inoculation of Pseudomonas isolate WPS90 with R. solani caused 58.8% disease reduction at 60 days of plant growth. At 75 days of plant growth, single inoculation with Pseudomonas isolate WPS3 caused 249% increase in shoot dry weight whereas inoculation with isolate WPS90 resulted in only 116% increase in shoot dry weight as compared to control uninoculated plants (Table 4). Coinoculation of isolates WPS3 and WPS90 with R. solani showed 238% and 156% gains in shoot dry weight, respectively. Maximum 88.2% disease control was observed on coinoculation of Pseudomonas isolate WPS3 with R. solani and only 58.8% disease reduction was observed on coinoculation of isolate WPS90. At 90 days of plant growth, single inoculation with Pseudomonas isolates WPS3 and WPS90 resulted in 131% and 47% increase of plant dry weight as compared to uninoculated control plants (Table 5) The coinoculation of Pseudomonas isolate WPS3 with R. solani showed only 115% increase in plant dry weight whereas coinoculation of Pseudomonas isolate WPS90 resulted in 98% increase in plant dry weight. Only 66.7% disease control was observed on coinoculation of Pseudomonas culture WPS90 with R. solani whereas coinoculation of Pseudomonas isolate WPS3 with R. solani caused 88.9% disease control (Table 5; Fig. 5). Thus, Pseudomonas strain WPS3 was found more effective in controlling the root rot disease caused by R. solani.Different microbial antagonists have been found to control the root rot caused by Rhizoctonia solani in different crops[45, 46]. Similarly, inoculation with P. aeruginosa and B. subtilis was found to significantly suppress root rot infection under green house as well as field conditions and enhanced the plant growth and yield in moongbean, wheat and maize[8]. Baig et al.[34] also reported that effects of bacterial inoculation on plant growth varied and plants showed stunted growth, root and shoot elongation or a neutral response. Three bacterial isolates increased root length while 14 isolates increased shoot length over the uninoculated control. An increase in fresh and dry matter was recorded by 16 bacterial strains. Sindhu et al.[14] reported plant growth promoting effects of fluorescent Pseudomonas sp. on coinoculation with Mesorhizobium sp. Cicer strain under sterile and “wilt sick” soil conditions in chick pea. The coinoculation resulted in enhanced nodulation by Mesorhizobium sp. and shoot dry weight was increased by 3.92 to 4.20 times in comparison to uninoculated controls. Recently, coinoculation of siderophore-producing Pseudomonas strain CP56 with Bradyrhizobium strain and R. solani showed maximum 275.8% increase in plant dry weight of green gram (Vigna radiata) at 60 days in comparison to control plants and also completely suppressed the root rot disease under pot house conditions[47]. In view of the potential application of these rhizosphere bacteria as biocontrol agents leading to suppression of plant diseases and due to their plant growth-promoting effects, the inoculation of plants with antagonistic rhizobacteria is a promising area of research to achieve maximum benefits in improvement of crop productivity.
5. Conclusions
- Pseudomonads and bacilli are predominantly found in the rhizosphere of cereal and legume crops. These rhizobacteria have immense potential for use as biofertilizer, biocontrol agent and/or in bioremediation due to their plant growth-promoting ability and antagonistic activity[9, 18, 29]. During these investigations, Pseudomonas and Bacillus isolates obtained from the rhizosphere of wheat were tested for antagonistic effect against pathogenic fungi R. solani and sixteen rhizobacterial isolates were found to inhibit the growth of R. solani. Growth inhibition zone varied from 6- by different rhizobacterial isolates. The culture filterate of selected antagonistic rhizobacterial isolates also showed growth inhibiton of the pathogenic fungi. Single inoculation with Pseudomonas isolates WPS3 and WPS90 resulted in 131% and 47% increase of plant dry weight as compared to uninoculated control plants, respectively at 90 days of plant growth. Whereas, coinoculation of Pseudomonas strain WPS3 with R. solani showed only 115% increase in plant dry weight and coinoculation of Pseudomonas strain WPS90 caused 98% increase in plant dry weight. Coinoculation of Pseudomonas isolate WPS3 with R. solani also caused 88.9% disease control. Thus, complex tripartite interactions between the rhizosphere bacteria, plant and pathogenic fungi showed variability in disease suppression and plant growth promotion in different Pseudomonas-inoculated treatments. The performance of these PGPR strains has to be tested under field conditions before their application as biocontrol agent in commercial agriculture.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML