-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Microbiology Research
p-ISSN: 2166-5885 e-ISSN: 2166-5931
2012; 2(1): 6-10
doi:10.5923/j.microbiology.20120201.02
Evaluation of Antimicrobial Activity of Anogeissus leiocarpus and Terminalia avicennioides against Infectious Diseases Prevalent in Hospital Environments in Nigeria
Abdullahi Mann
Department of Chemistry, Federal University Technology, Minna, P. M. B. 65, Niger State, Nigeria
Correspondence to: Abdullahi Mann, Department of Chemistry, Federal University Technology, Minna, P. M. B. 65, Niger State, Nigeria.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
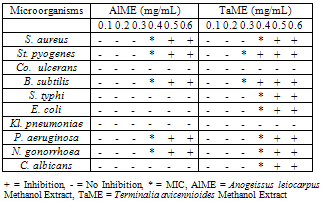
The crude methanol extracts of the two plants selected on the basis of ethnobotanical uses were phytochemically screened and found to contain saponins, steroids, tannins, terpenes, anthraquinone and carbohydrates. The crude methanolic extracts exhibited broad growth inhibition against Staphylococcus aureus, Streptococcus pyogenes, Bacillus subtilis, Salmonella typhi, Escherichia coli, Pseudomonas aeruginosa, Neisseria gonorrhoea and Candida albicans. The Minimum Inhibition Concentration (MIC) of Anogeissus leiocarpus methanol extract was found to be 0.4 mg/mL against S. aureus, St. pyogenes, P. aeruginosa and N. gonorrhoea, while B. subtilis has the MIC of 0.3 mg/mL. The rest of the microorganisms were resistant to Anogeissus leiocarpus methanol extract. Terminalia avicennioides methanol extract has the MIC of 0.4 mg/mL on S. aureus, S. typhi, E. coli, P. aeruginosa, N. gonorrhoea and C. albicans. Both S. pyogenes and B. subtilis have the MIC of 0.3 mg/mL against Terminalia avicennioides methanol extract. The rest of the microorganisms were resistant. Both Corynebacterium ulcerans and Klebsiella pneumoniae were resistant to the two plant extracts. The present investigation justify the use of these extracts in the treatment of infectious diseases particularly those caused by S. pyogenes having the highest zone of inhibition as well as the lowest MIC (0.3 mg/mL) for the two plants. Further purification of the most promising extracts is currently being undertaken.
Keywords: Antimicrobial Activity, Anogeissus leiocarpus, Terminalia avicennioides, Methanol Extract
Cite this paper: Abdullahi Mann, Evaluation of Antimicrobial Activity of Anogeissus leiocarpus and Terminalia avicennioides against Infectious Diseases Prevalent in Hospital Environments in Nigeria, Journal of Microbiology Research, Vol. 2 No. 1, 2012, pp. 6-10. doi: 10.5923/j.microbiology.20120201.02.
Article Outline
1. Introduction
- Infectious diseases are one of leading cause of premature death. Infective diseases account for approximately one-half of all death in tropics[1]. Resistance to antimicrobial agents is a major global public health problem[2], partly due to indiscriminate use of antibiotics[3]. Despite seeming progress made in the development of antimicrobial agents, occurrence of drug resistant microorganisms and the emergence of unknown disease-causing microbes, pose enormous public health concerns[2]. Staphylococci and Streptococci are among the organisms that cause respiratory infections. C. albicans is the common commensals of the gastro-intestinal and urogenital tracts of human[4], as well as candidiasis in women. Pseudomonas species are particularly noted for causing urinary tract infections and sepsis which are currently resistant to virtually all the older antibiotics[5]. New therapeutic agents are of great demand. Many infectious diseases are known to be treated with herbal medicines throughout the human civilization. Even today, plant materials continue to play major role in primary health care andhigher plants have been shown to be potential sources for the new anti-microbial agents[6]. Indigenous plants are reservoirs of various metabolites and provide a limitless source of important chemicals that have diverse biological properties[7]. Many of modern day drugs have their origin in traditional plant medicine[8]. In the area of anti-infectives (anti-bacterial,-fungal, -parasitic, and -viral), about 70 % are naturally derived[9]. The screening of plant extracts for antimicrobial activity has shown that higher plants represent a potential source of novel antibiotic chemotypes.The therapeutic efficacies of many indigenous plants for treating ailments have been described by practitioners of traditional herbal medicines[10,11]. The practice of herbal medicine which employs plants as its major components is an integral part of traditional and culture of Africans[12]. Most therapeutic attributes of medicinal plants are traced to the plant constituents and the medicinal actions of these constituents are unique to particular plant species or family. In particular, the antimicrobial activity of plant extracts has formed the basis of many applications including traditional medicine, pharmaceuticals, natural and orthodox therapies.
1.1. Extraction of the Crude Extracts
- Two kilogrammes (2 kg) each of stem bark of A. leiocarpus and root bark of T. avicennioides were powdered using wooden mortar and pestle, and extracted by percolation (cool maceration) for 72 h at room temperature with 70% methanol (3 X 2.5 mL: the methanol extracts were filtered and the process was repeated three times for exhaustive extraction, to ensure that no metabolites were left in the residues). All the filtrates for each set were combined and then concentrated under low pressure to dryness at 35℃ using a rotavapor (W240N). The dried methanol extracts obtained from each plant were air-dried then packed in glass bottles with proper labelling for future reference. The extracts were refrigerated and kept away from light (wrapping with aluminium foil prior to further processing). The crude extracts of each plant species was tested for antimicrobial activity.
1.2. Phytochemical Analysis of Extracts
- The plant extracts were phytochemically screened using standard techniques for the detection of carbohydrates, saponins, tannins, terpenoids, glycosides and alkaloids[33-35].
1.3. Determination of Antimicrobial Activity
- The standard and local strains of microorganisms: Staphylococcus aureus, Streptococcus pyogenes, Corynebacterium ulcerans, Bacillus subtilis, Salmonella typhi, Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, Neisseria gonorrhoea and Candida albicans were obtained from the Department of Medical Microbiology Ahmadu Bello University Teaching Hospital Zaria. Bacteria cultures were checked for purity and maintained in a slant of Blood agar base. The Paper disc diffusion technique as described by Bauer-Kirby Method[36] was used in determination of the antimicrobial activities of the extracts. Stock solutions of crude methanolic extracts were prepared by dissolving 1 mg of each extract in 100 ml of the dimethyl sulphoxide (DMSO). This solution was used in the determination of antimicrobial activities of the extracts. For the determination of Minimum Inhibition Concentration (MIC) of crude Anogeissus leiocarpus Methanol Extract (AlME) and Terminalia avicennioides Methanol Extract (TaME) extracts against the microorganisms. Solution concentrations of 0.1, 0.2, 0.3, 0.4, 0.5, 0.6 mg/mL were prepared for each extract by serial dilution. Blood agar base was used as the growth medium for the microorganisms. The plates containing the sterile medium were seeded with the microorganisms by the spread plate technique and were then left for half an hour to dry. Filter paper discs were cut and sterilized for 1 hr[36], and then soaked in the solution of the extract concentrations prepared and dried at 45oC for 1 hr. The dried paper discs were then planted on the blood agar plates seeded with test microorganisms. The plates were then incubated at 37℃ for 24 hrs in the case of bacteria species, before they were examined for the zone of inhibition of growth. Tetracycline (0.5 mg/mL) was used as reference or positive control while DMSO without sample was used as negative control. While the agar tube dilution method was applied for determination of the antifungal activity against Candida albicans. Miconazole was used as positive control. The diameters of the zones of inhibition of growth were measured and recorded in millimetres.
2. Results and Discussion
2.1. Phytochemical Analysis of the Extracts
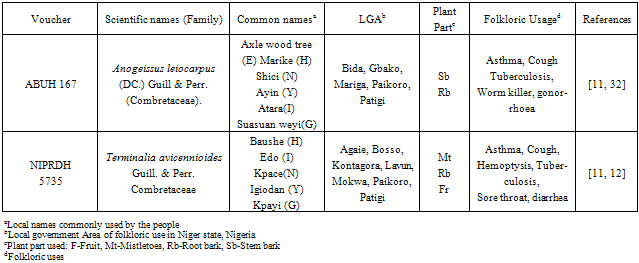
- The results of the phytochemical analysis as shown in Table 2 prominently indicate the presence of saponins, steroids, tannins, terpenes, anthraquinone and carbohydrates. From the previous preliminary phytochemical screening of stem bark extracts of A. leiocarpus indicated presence of tannins, saponins, phenols and anthraquinones[18] only, but the present screening of the same part showed also the presence of steroids, terpenes and carbohydrates (Table 2). The root bark of T. avicennioides indicates the presence of anthraquinone, saponins, steroids, tannins and terpenes. The percentage yields for the A. leiocarpus and T. avicennioides are 4.8 and 15.4 % respectively.
|
|
2.2. Antimicrobial Activity of the Extract
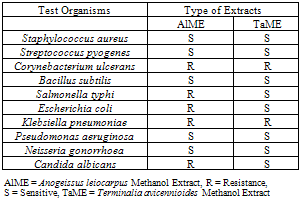
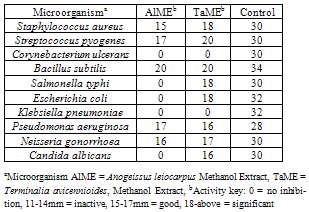
- The antimicrobial activities of crude methanol extract from AlME and TaME were determined using some standard and local strains of microorganisms as shown in Tables 3 and 4. The crude methanol extract of T. avicennioides root bark was sensitive to all organisms except Co. ulcerans and Kl. pneumoniae. While S. aureus, St. pyogenes, and B. subtilis, N. gonorrhoea and P. aeruginosa were sensitive to the crude methanol extract of A. leiocarpus stem bark the rest were resistant (Table 3). The crude methanol extract of T. avicennioides root bark showed significant antibacterial activity against S. aureus, S. typhi, E. coli, St. pyogenes and B. subtilis by showing zone of inhibition 18,18, 18, 20 and 20 mm, respectively, but exhibited good inhibition against N. gonorrhoea (16 mm) and P. aeruginosa (17 mm). On the other hand the crude methanol extract of the A. leiocarpus stem bark was significantly active against only one bacterium, B. subtilis (20 mm), but also showed good inhibition of growth of few bacteria (Table 4). Only extract of T. avicennioides exhibited good inhibition against C. albicans. The MIC of AlME was found to be 0.4 mg/mL against S. aureus, S. pyogenes, P. aeruginosa and N. gonorrhoea, while B. subtilis has the MIC of 0.3 mg/mL (Table 5). TaME has the MIC of 0.4 mg/mL on S. aureus, S. typhi, E. coli, P. aeruginosa, N. gonorrhoea and C. albicans. Both S. pyogenes and B. subtilis have the MIC (0.3 mg/mL) (Table 5). The results of the present investigation clearly demonstrated the antimicrobial potentials of the crude methanol extracts of both A. leiocarpus and T. avicennioides. The two extracts from these plants possess significant in vitro antimicrobial activities against some of the bacteria implicated in the pathogenesis of human infections. Some infections such as: respiratory tract inflammations caused by Pseudomonas species and typhoid fever caused by Salmonella species, are often difficult to combat, but the growth of these organisms were greatly inhibited by extracts from both plants as shown in Table 3 and 4. While E. coli incriminated as the causative agent of gastro-intestinal and also causes infections in the lungs especially in immunodeficient patients[37] was also susceptible to the extract from T. avicennioides. It is worthy to note that the antimicrobial activity found in Anogeissus species in the Sudan has been attributed to 3, 3, 4’ –tri-O-methylflavellagic acid extracted from the bark[15]. However, the activities of the stem bark extract of A. leiocarpus against N. gonorrhoea and B. subtilis as well as those of root bark of T. avicennioides against S. pyogenes, N. gonorrhoea and C. albicans have just been reported. It is a common practice among the traditional healers in Niger State to prepare an infusion of A. leiocarpus and T. avicennioides separately to relieve upper respiratory tract infections, fever, cough, TB and stomach pains. The susceptibility of these microbes to the extracts of these plants may be a pointer to their potentials as drugs that can be used against these organisms.
|
|
|
3. Conclusions
- The current findings lend credence to the traditional use of these plants as medicines for infectious diseases particularly those caused by the test organisms susceptible to the extracts. The present results for both plants indicate significant antimicrobial potentials and this suggests that traditional medicine could be used as guide in the continuous search for new antimicrobial agents. It also forms the basis for further investigation on purification and structural determination of the most promising constituents for in vivo evaluation of toxicity of these plants in animal and human studies.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML