-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Microbiology Research
p-ISSN: 2166-5885 e-ISSN: 2166-5931
2012; 2(1): 1-5
doi:10.5923/j.microbiology.20120201.01
The Antimicrobial Effect of Some Selected Nigerian Chewing Sticks on Clinical Isolates of Candida Species
1Department of Biological Sciences, Redeemer’s University, Mowe, Ogun State
2Deparment of Microbiology, OlabisiOnabanjo University, Ago-Iwoye, Ogun State
Correspondence to: Osho A., Department of Biological Sciences, Redeemer’s University, Mowe, Ogun State.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Extracts of selected chewing sticks ( Fagarazan thoxyloides, Anogeissus leiocarpus and Diste moranthus benthamianus ) obtained in Ibadan metropolis, were assayed for their antifungal activities against Candida sp(C. albicans, C. kruseiand C. tropicalis). Aqueous and ethanolic extractions were carried out to obtain the active ingredients of the chewing sticks. The agar diffusion method was used to assay the antifungal activity of the extracts. Both the aqueous and ethanol extracts of all the chewing sticks exhibited inhibitory activity on the growth of C. albicans, C. krusei and C. tropicalis. The zones of inhibition produced by the different extracts against the Candida species were significantly different at standard of mean. Data from the present study have shown the chewing sticks as a potential candidate for the production of dentifrice and other natural products for oral hygiene and treatment of tooth problems.
Keywords: Nigerianchewing Stick, Clinical Isolates, Antifungal Activities, Inhibitory Activity, Aqueous Extract, Candida
Cite this paper: Osho A., Adelani O. A., The Antimicrobial Effect of Some Selected Nigerian Chewing Sticks on Clinical Isolates of Candida Species, Journal of Microbiology Research, Vol. 2 No. 1, 2012, pp. 1-5. doi: 10.5923/j.microbiology.20120201.01.
1. Introduction
- The use of chewing sticks has been documented since ancient times. This kind of tooth brushing has been used by the Babylonians some 7000 years ago[1]. In many traditional cultures, there are no plastic-bristle brushes, rather, the use of herbal chewing sticks for relieving dental problems is common. The cleansing efficacy of chewing sticks is attributed to the mechanical effects of its fibers, release of beneficial chemicals or a combination of both[2]. Some African chewing sticks are also reported to contain fluoride ions, silicon, tannic acid, sodium bicarbonate and other natural plaque inhibiting substances that can reduce bacterial colonization and plaque formation[3]. The repeated process of using chewing sticks releases fresh sap containing fluoride, which seems to wet the tooth enamel and adequately reach caries susceptible sites and contribute towards caries prevention[4]. Tannin exerts an astringent effect on the mucous membrane, thus reducing the clinically detectable gingivitis[5]. Tannins also inhibit the action of glucosyltransferase thus reducing plaque and gingivitis[6]. Resines forms a layer over the enamel and thus protects against caries. Alkaloids exert bactericidal effect in the oral cavity[7]. Essential (volatile) oils possess characteristic aroma and exert antiseptic action[8]. The mild bitter taste sulfur compounds havea bactericidal effect[10]. Vitamin C is antioxidant and helps in the healing and repair of tissues. Sodium bicarbonate has mild abrasive properties and is, thus, used as a dentifrice in addition to having a mild germicidal action[11,12]. The high concentrations of chloride inhibit calculus formation[13] and help in removing stains from the teeth [8] Calcium saturation of saliva inhibitsdemineralization and promotes re- mineralization of tooth enamel[6].A great number of these plant species have related medicinal properties that may be antibacterial[4,3]. D. benthamianusis rich in flavonoid compounds[14], such as oxyayain A, oxyayain B, Ayanin and Distemonanthin. These components have been implicated in antitumor activity, antioxidative activity[15], anti-adrenergic activity[16] and treatment of bacterial, fungal and viral infection[14]. Anogeissusleiocarpus is also another plant species used in traditional medicine as a remedy for many ailments of livestock and man, which include helminthosis, schistomiasis, leprosy, diarrhea and psoriasis[17,18]. In addition to these applications, Hollist[19] reported that A. leiocarpusis one of the major plants commonly used as chewing stick in Nigeria. Fagarazanthoxyloides is also widely distributed in African countries. The root-bark extract is used in treating elephantiasis, toothache, sexual impotence, gonorrhoea, malaria, dysmenorrhoea and abdominal pain [20-22]. Many studies have demonstrated the antimicrobial, anticarries, anti- periopathic and antifungal properties of both aqueous and ethanolic extracts of various chewing sticks[23-26]. There are documented reports on the antimicrobial activity of A. leiocarpuson oralmicroflora.[15]reported the antimicrobial effect of its root extract on Staphylococcus aureus and Pseudomononasaeroginosa.[27]documented the antibacterial activity of its bark extract on Bacteriodesgingivalisand Bacteriodesmalaninogenicus. Workers in West Africa have also reported the anti-sickling and antimicrobial activity of the extracts of Fagarazanthoxyloides[28]. Water extracts from the plant showed activities against bacteria significant to periodontal disease[30,24]. The anthelmintic activity of the methanolic extract of the root-bark of F. zanthoxyloides was also reported[31], and it is a very popular anthelmintic amongst the various tribes in Uganda. It has also been found that the alcoholic extracts of the root-bark possesses considerable antibacterial activity[32]. An anti-sickling agent [29] and an anti-inflammatory amide were isolated from the plant[33]. Therefore, this paper examines the inhibitory activity of some selected Nigerian chewing sticks extracts against selected clinical isolates of Candida species.
2. Materials and Methods
- Collection of MaterialsThe chewing sticks (Fagarazanthoxyloides, Anogeissusleiocarpus and Distemoranthusbenthamianus) used were obtained from King’s market (Oja Oba) in Ibadan, Oyo state, Nigeria. They were authenticated by a Botanist at the Department of Botany, OlabisiOnabanjo University, Ago- Iwoye, Ogun State, Nigeria.Yeast strainsThe modified methods of[34] was employed for the isolation of Candida species. Candida strains were obtained from the oral cavity of healthy young adult from the students of OlabisiOnabanjo University aged between 18 and 26 years. Individual with medical history for candidiasis were selected. Sterile swabs were used to collect clinical specimen from saliva, tongue dorsum, palate mucosa, dental biofilm and denture. The swab was immediately immersed in a tube containing sterile saline and the tube agitated for 30 seconds. 100 µl from this clinical specimen was then diluted 10-fold and 100 fold in sterile saline. An aliquot of 100 µl from the diluted sample was inoculated in plate containing Sabouraud dextrose agar (Merc Germany), 0.1 mg/ml chloramphenicol was added to each and duplicates were setup. The plates were incubated at 37 0C for 48 h. After incubation, identification of the Candida species was carried out by standard-taxonomic methods which include germ tube production, microscopic appearances on cornmeal agar with Tween-80, pigment production on chromogenic mediun, chlamydospore production and colony morphology. This identification was confirmed with the test kit, API 32 C AUX (bioMerieux, Marcy-l’Etoile, France).Plant preparationThe test plant parts were washed in distilled water and air dried at room temperature for two weeks. The dried plant parts were chopped into bits before blending with an electric blender. The blended chewing stick samples was sterilized by autoclaving at 1.02Kgm-2 pressure at 1210C for 10 minutes and allowed to cool to room temperature before dispensing aseptically into 250ml Erlenmeyer flask and tapered.Extraction5g each of the chewing stick samples were separately soaked in 100ml of distilled sterilized water and 95% ethanol respectively. This was allowed to stand for 7 days for extraction of the active ingredients. The solvents were then filtered using Whatman No1 filter paper. The extracts were then kept in fridge prior to use.Antifungal AssayAgar-well diffusion method was used for the assay. The Candida strains were sub-cultured to obtain a more vigorous population. The fresh stocks were used to seed 20ml Potato Dextrose broth. Spore suspension of 105–107 cells was prepared and 0.5ml was used to inoculate prior prepared sterile PDA plates. The 6 mm diameter cork borer was used to make well on the plates for introduction of different test plants extracts. The plates were incubated and the zone of inhibition was measured in mm after 24 and 48 hours of growth. A control experiment was set up by using sterile distilled water and ethanol differently in place of different extracts of the test plants.
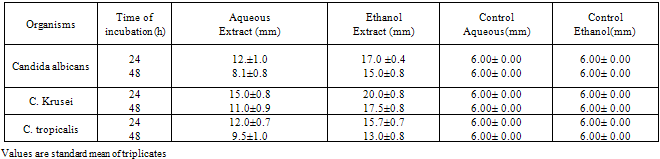
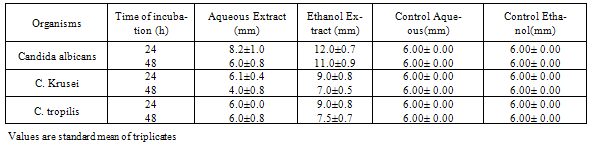
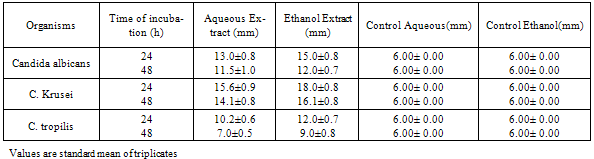
3. Results
- The results of the antifungal assay are presented in Table 1, 2 and 3. From the present data, it is evident that both the aqueous and ethanol extracts of all the test plant parts exhibited inhibitory activities on the growth of C. albicans, C. krusei and C. tropicalis.
|
|
|
4. Discussion and Conclusions
- The results of the antimicrobial assay depict that the aqueous and ethanol extracts of the plant parts demonstrated inhibitory activity on the growth of the three tested fungi. However, these results are contrary to earlier reports [35; 36,37] who indicated that there was no activity of A. leiocarpusextract on C. albicans. The contradiction in the results to the earlier reported research could be as result of differences in the concentration of active ingredient in the chewing stick extracts. On the other hand, it could also be attributed to the microbial load used for the anti-fungal assay. All these are capable of bringing about variations in the result. Extracts of F. zanthoxyloideswere completely active against all the fungi tested. The zone of inhibition produced by Fagarazanthoxyloides against C. krusei was more pronounced than the rest of the test fungi. The result is quite contradictory to that of[38] who reported on the lack of effect of F. zanthoxyloideson the adherence and growth of the oral opportunist pathogen and that the extracts of F. zanthoxyloideswere completely inactive against all the microbes tested. This could be due to the fact that they tested the extracts against bacterial oral pathogens.A large number of constitutive plant compounds have been reported to have antimicrobial activity. Well known examples include phenols, unsaturated lactones, saponnins, cyanogenic glycosides and glucosinolates[39,40]. Various chemicals such as alkaloids, tannins, saponnins, cyanoglycosides, terpenoids, oleic and stearic acids which are naturally present in plants have been implicated in the conferment of antimicrobial activities on the plant containing them [39, 40, 41].The presence of these phytochemicals in the investigated test plant parts would be responsible for the demonstrated antimicrobial activity of the different plant extracts. Furthermore, these plant secondary metabolites must have been present in a significant amount in the investigated parts of the plants in order to have been able to confer antimicrobial activity on the extracts of this plant. Previous reports have indicated that the root of A. leiocarpusis often used as chewing stick [25,15]. Paradoxically, this study also showed that the stem also contained active agents against the tested oral pathogens, and thus could be used in the absence of the root of this plant. Hence, higher plants, as sources of medicinal compounds continue to play dominant role in maintenance of human health since antiquities. Over 50% of all modern clinical drugs are of natural product origin [42] and natural products play an important role in drug development programs of the pharmaceutical industry [43,44] In this regard, the use of plants in the production of dentifrice and natural chewing gums for oral hygiene and to treat toothache, gingivitis and periodontal disease has been reported by Kerry [45]. Data from the present study have implicated the plants parts as a potential candidate in such application. It is hoped that this discovery would be utilized to better the oral health of Africans whose landscape is replete with these plants.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML