-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Microbiology Research
p-ISSN: 2166-5885 e-ISSN: 2166-5931
2011; 1(1): 8-16
doi: 10.5923/j.microbiology.20110101.03
Evaluation of Kinetic Parameters of Endosulfan Biosorbed on Biosorbent Prepared from Aspergillus nidulans
Deepika Dave 1, Anil Kumar Dikshit 2, 3, 4
1Process Engineering and Applied Science, Dalhousie University, Halifax, NS B3H 4R2, Canada
2Centre for Environmental Science and Engineering, Indian Institute of Technology Bombay, Powai, Mumbai, 400076, India
3School of Civil Engineering, Survey and Construction, University of KwaZulu-Natal, Durban, 4041, South Africa
4School of Civil and Environmental Engineering, Nanyang Technological University, 639798, Singapore
Correspondence to: Anil Kumar Dikshit , Centre for Environmental Science and Engineering, Indian Institute of Technology Bombay, Powai, Mumbai, 400076, India.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
The biosorption of endosulfan from aqueous solution on biosorbent prepared from fungal culture Aspergillus nidulans(ANS) was studied in a batch system with respect to different initial concentrations, adsorbent size, agitation and interruption. The kinetic parameters of sorption process such as equilibrium times and different diffusion coefficients were evaluated. The pseudo-first-order, pseudo-second-order kinetic model and the intraparticle diffusion model were used to describe the kinetic data and the rate constants were estimated. The experimental data fitted very well the pseudo-second-order kinetic model and also followed the intraparticle diffusion model up to 12 hrs. The intra-particle diffusion along with the external diffusion was determinant of the overall rate of the biosorption of endosulfan on to ANS biosorbent.
Keywords: Aspergillus Nidulans, Biosorbent, Biosorption, Endosulfan, Film Diffusion, Kinetics, Pore Diffusion
Cite this paper: Deepika Dave , Anil Kumar Dikshit , "Evaluation of Kinetic Parameters of Endosulfan Biosorbed on Biosorbent Prepared from Aspergillus nidulans ", Journal of Microbiology Research, Vol. 1 No. 1, 2011, pp. 8-16. doi: 10.5923/j.microbiology.20110101.03.
Article Outline
1. Introduction
- Endosulfan, a hydrophobic chemical, is a broad-spectrum insecticide and acaricide first registered for use in the United States in 1954 to control agricultural insect and mite pests on a variety of field, fruit, and vegetable crops. Technical-grade endosulfan is composed of two stereochemical isomers, α-endosulfan and ß-endosulfan, in concentrations of approximately 70% and 30%, respectively. The chemical is marketed by many different companies and under a variety of names including: Agrosulfan; Aginarosulfan; Banagesulfan; Cyclodan; Endocel; Endoson; Endonit; Endomil; Endosol; Endostar; Endodaf; Endosulfer; E-sulfan; Endorifan; Hildan; Redsun; Seosulfan; and Thiodan. It is also comes under the list of most stable known pesticides. It has been detected in the atmosphere, soils, sediments, surface and rain waters, and food stuffs[1]. It has extreme toxicity towards fish, aquatic invertebrates and mammalian[2,3]. Its presence in the environment may be the cause of several health effects including genetic disorders[4] and neurological disorders[5]. These health and environmental concerns have led to an interest in detoxification of endo-sulfan from the environment.Many international organizations have enforced stringent limits of pesticides in water and food in order to prevent human risk and environmental pollution. As per USEPA (1999), the guideline value for endosulfan and metabolites in drinking water is 0.22 µg/L and 0.1 to 0.2 mg/L in agricultural products. According to Bureau of Indian Standards (BIS), no pesticide should be present in natural waters and should not exceed 0.005 mg/L in surface waters[6]. Worldwide lots of efforts have been put for removal or reduction of pesticides from water environment. Conventional methods for the removal of pesticides are found to be either uneconomical or insufficient[7-11]. Biosorption comprises binding of pollutants(solutes, colloids or suspensions) to the biomass by a process(via physico-chemical mechanisms such as adsorption or ion exchange) which does not involve metabolic energy or transport, although this process may also occur simultaneously where live biomass is used. Biosorption is generally used for the removal of heavy metals from aqueous phase[12-15]. Removal of organic and other pollutants from aqueous phase by biosorption has already been attempted and documented in literature[16-21].In present study, biosorption technique was attempted for removal of endosulfan from water environment by biosorbent prepared from fungal culture, Aspergillus nidulans. The objective of the present work was to find out the process that is controlling the rate of sorption. The rate limiting step was evaluated to find the relative importance of film diffusion(external mass transfer) and pore diffusion(intra-particle diffusion) and also to decide which of the two dictates the overall rate of adsorbate transfer onto biosorbent sites. The rate limiting step was determined using a number of approaches viz. based on film and pore diffusion, based on external mass transfer, based on the effect of initial adsorbate concentration, based on the effect of adsorbent size, based on the effect of agitation and based on interruption on test.
2. Materials and Methods
2.1. Microorganism and its Growth Conditions
- Fungal culture, Aspergillus nidulans (NCIM 1211) was procured from National Collection of Industrial Micro- organisms (National Chemical Laboratory, Pune, India) and used to prepare biosorbent for removal of endosulfan. Slants and petri dishes were made using fungal potato dextrose agar medium (1 L distilled water, 200 g peeled potatoes, 20 g dextrose, 0.1 g yeast extract, 20 g agar) and potato dextrose broth (1 L distilled water, 200 g peeled potatoes, 20 g dextrose, 0.1 g yeast extract) was used as liquid medium. The liquified agar was pored into sterilized culture tubes and petri plates, and allowed to solidify. The culture were then streaked on slants and petri plates and incubated in a bacteriological incubator (Trishul Equipments, Mumbai, India) at 37℃ for 4 days. After 96 h, colonies with dark green color started forming on the potato dextrose agar (Figure 1a). The microscopic view of the long aerial hyphae of Aspergillus nidulans using Zeiss image analysis microscope (model Axion Star Plus, Germany) is shown in Figure 1b. The subcultures were preserved in the cold room at 4℃.
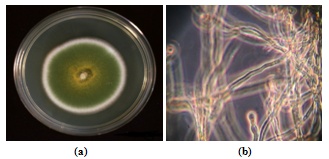 | Figure 1. Aspergillus nidulans (a) Colony with dark green colour on potato dextrose agar (b) Long aerial hyphae observed under the Zeiss image analysis microscope |
2.2. Preparation of Biosorbent
- Aspergillus nidulans was grown in an autoclaved NCIM 44 Potato dextrose medium with corresponding fungal spores (approximately 10% of the volume of medium). Test flasks were placed on rotary platform incubator shaker (Trishul equipments, Thane, Mumbai) maintained at 37℃ and 250 rpm for 4 days. Spore inoculum was prepared under sterile conditions in 0.05% in Tween-80 using freshly grown and sporulating mat on potato dextrose agar plates. The mycelia pellets were harvested through filtering. The biomass was then washed repeatedly with distilled water to remove the growth medium adhering on its surface. The mycelia pallets used in this study were autoclaved at 121℃ for 20 minutes. The biomass was then dried in an oven at 103℃ for 12 hrs and ground with a laboratory grinder and sieved to get particle size between 0.15–0.3 mm.
2.3. Chemicals
- All chemicals and reagents used were of analytical grade (AR). Technical grade endosulfan of 96.14% purity was obtained from M/s Vijiyalaxmi Insecticides and Pesti cides Limited(Andhra Pradesh, India). N-Hexane and Acetone were purchased from Merk India Limited (Mumbai, India). Distilled water was used for making synthetic samples. Before every experiment, all glassware were cleaned with mixture of dilute chromic acid and soap solution followed by through washing with tap water and distilled water. Stock solution of endosulfan was prepared in acetone at a concentration of 100 to 1000 mg/mL. All solutions were stored in the dark at 4℃ prior to use.
2.4. Extraction of Endosulfan from Water Samples
- The flasks containing endosulfan was separated from the fungal biosorbent by filtering through filter paper no. 201. Prior to filtration, the filters were washed with 300 mL of distilled water to remove any leachable materials. About 40-45 mL of extracted solution was filtered through the filter paper to achieve biosorption equilibrium of filter paper with the solution. This portion of filtrate was discarded. The subsequent filtrate was then extracted by n-Hexane as prescribed in the following paragraph and collected for GC analysis in teflon sealed septum bottles.Extraction of endosulfan from water was done by liquid-liquid partition method. Representative sample of 2 mL of aqueous solution spiked with endosulfan was extracted in a 20 mL test tube. Extraction was done three times with 2 mL, 2 mL, and 2 mL of n-Hexane, respectively. During the extraction process, the sample-hexane mixture was shaken for 60 seconds in multi tube vertex mixer(Trishul equipments, Thane, Mumbai). Then, allowed to settle for 1 minute. Hexane extract was collected in 20 mL test tube and made to 6 mL with n-Hexane(some hexane was lost due to evaporation during transfer of solvent). 1 mL from 6 mL extracted hexane was transferred to Teflon sealed septum bottles. 2 µL of extracted sample was used for injecting into GC.
2.5. Analytical Procedures
- Gas Chromatograph (Agilent Technologies, model GC- 6890N) with electron capture detector and Agilent HP-5 column(0.53 mm internal diameter, 1.5 µm film thickness and 30 m length) was used for endosulfan n-Hexane extracts. Temperature of column, injector and detector was maintained at 280℃, 250℃ and 300℃ respectively. Nitrogen (99.9% purity) was used as carrier gas at a flow rate of 30 mL/min.
2.6. Kinetic Experiments
- All batch sorption experiments were carried out using synthetic samples in distilled water at room temperature with constant stirring using mechanical shaker (Trishul Equipments, Thane, Mumbai). A high agitation speed of about 240-250 rpm was maintained to keep the sorbents in suspension during the experimental studies. Screw-top flasks (Borosil, India) of 100 mL capacity were used in the kinetic experiments. Synthetic water sample (50 mL) of 1 mg/L concentrations of endosulfan was taken in separate sets of flasks and the adsorbents were added to these at a suitable dose. All experiments are carried out for 14 hrs and Samples were collected at 30 minutes interval up to first 3 hours; every one hour interval up to 12 hrs; every 2 hours interval up to 14 hrs. All flasks were withdrawn from the system after the required reaction time and biosorbent was separated by gravity filtration. Representative samples (5 mL) were taken from the flask, extracted and analyzed for pesticide as per the method outlined in earlier sections. The sorption kinetics was studied at different initial concentrations of endosulfan 1, 2 and 4 mg/L. The time required by endosulfan to reach the equilibrium condition was determined using the kinetic profiles. The effect of biosorbent size on sorption kinetics was studied by taking four different size ranges viz 0-0.075 mm, 0.15-0.30 mm, 0.425-0.60 mm and 0.60-0.85 mm. The effect of agitation speed on removal of endosulfan was also studied for two different agitation speeds of 175 rpm and 200 rpm.
2.7. Interruption Test
- A series of 16 screw cap conical flasks of 100 mL were taken. The reaction mixtures containing 50 mL of distilled water spiked with 1 mg/L of endosulfan in flasks and 1 g/L of biosorbent were agitated in the mechanical shaker at an agitation speed of 250 rpm at room temperature. Interruption tests were conducted in eight screw cap conical flasks and eight flasks were maintain uninterrupted. After 15 min of shaking, one sample flask was taken out of the shaker, biosorbent was removed from the 1st sample flask and interrupted for a period of 30 min. After the completion of 30 min of interruption the separated biosorbent was reintroduced into the 1st sample flask, and the first sample flask was finally withdrawn after shaking for another 15 min. Therefore, the first sample was agitated for 30 min(15+15 min) with an interruption of 30 min. Similarly, the second sample flask was withdrawn for an interruption of 30 min after initial agitation of 30 min. The second sample flask was again agitated for another 30 min and withdrawn completely. Therefore, the second flask was agitated for 1 hr with an interruption of 30 min. Similarly, 3rd, 4th, 5th, 6th, 7th and 8th sample flasks were withdrawn for an interruption of 30 min after an initial agitation of 90, 150, 210, 270, 330 and 390 min respectively and reintroduced for another period of 30 min agitation. All the flasks were interrupted for 30 min and had a total agitation of 30, 60, 120, 180, 240, 300, 360 and 420 min sequentially from 1st to 8th flask. The other eight flasks on the shaker were withdrawn completely after 30, 60, 120, 180, 240, 300, 360 and 420 min of shaking without any interruption. The samples were collected from all the 16 flasks and initial concentrations of the sample were analyzed for endosulfan concentration.
3. Results and Discussion
3.1. Biodsorption Kinetic Study and Modelling
- The removal kinetics of endosulfan by the 1 g/L of ANS biosorbent (size 0.15-0.3 mm) for different endosulfan concentrations are shown in Figure 2. More than 60-70% of endosulfan was biosorbed within 5-6 hrs of reaction time in case of initial concentration of 1 mg/L. The removal rate was diminishing after 9 hrs of contact time and accounted only for 5.40% between 9 to 12 hrs. After 12 hrs of reaction time, the kinetics profiles gradually became horizontal indicating the final equilibrium. The removal of endosulfan beyond 12 hrs of reaction time was very slow; hence an equilibrium time of 12 hrs was standardized for the rest of the experiments. Figure 2 also shows that the initial percentage biosorption decreased 35.7%, 30.2% and 24.0% as the concentration was increased from 1 mg/L, 2 mg/L and 4 mg/L respectively.
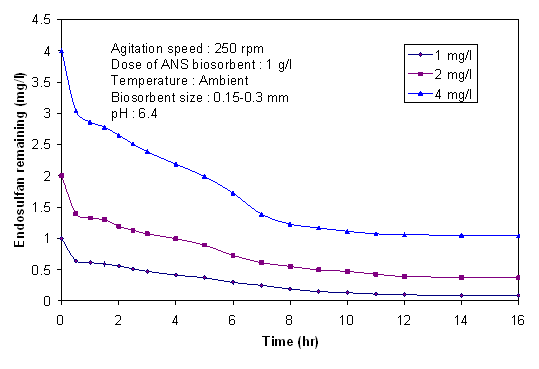 | Figure 2. Change of concentration of endosulfan with time at different initial concentrations |
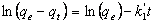 | (1) |
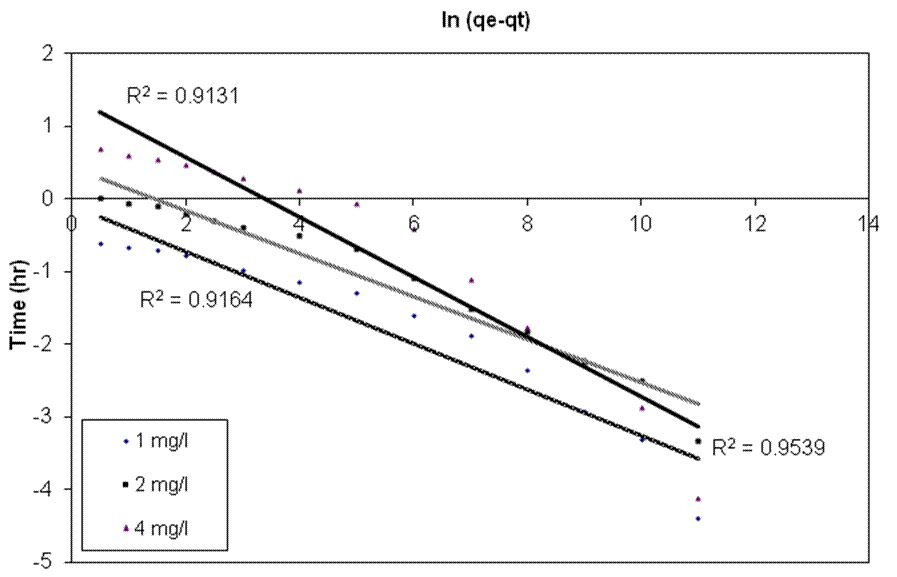 | Figure 3. Linearized pseudo first order kinetic plots for the biosorption of different concentrations of endosulfan onto ANS biosorbent |
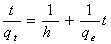 | (2) |
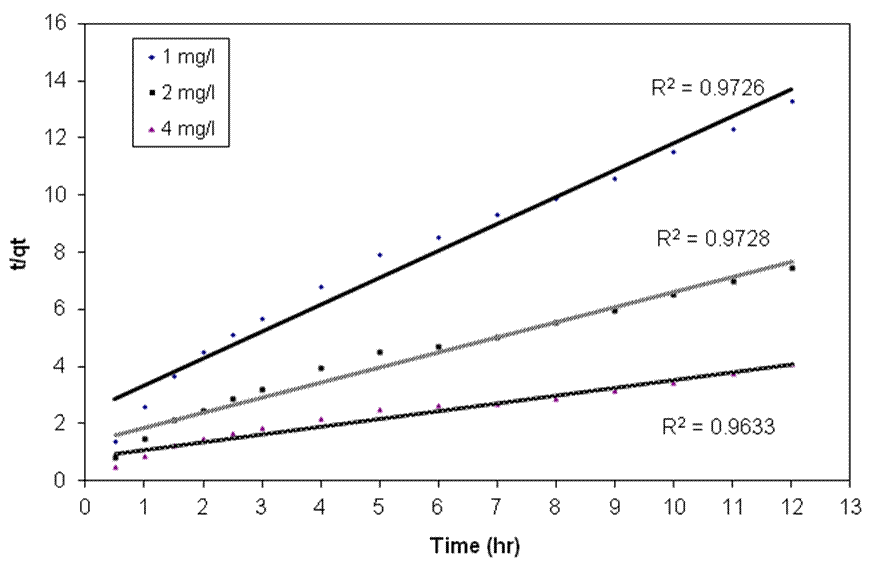 | Figure 4. Linearized pseudo second order kinetic plots for the biosorption of different concentrations of endosulfan onto ANS biosorbent |
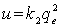 | (3) |
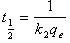 | (4) |
3.2. Calculation of Film and Pore Diffusion Coefficient
- The film diffusion coefficient (Df) and pore diffusion coefficient(DP) are two important parameters of the kinetic study of the adsorption process. The diffusion coefficients were calculated by method based on adsorption rate proposed by Helfferich[34], which assumes the spherical geometry of adsorbent particles and uses the following equations to estimated the film and pore diffusion coefficients. By assuming a spherical geometry for the adsorbent particles, Helfferich[34] derived the following half time equations:
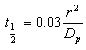 | (5) |
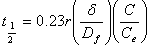 | (6) |
|
3.3. External Diffusion Analysis
- External diffusion, internal diffusion (or intraparticle diffusion), and actual adsorption are the three stages for the adsorption process on a porous adsorbent[39]. Rapid diffusion and adsorption generally occur in the macro-pores and the remaining slow approach to equilibrium occurs in the micro-pores. The concentration decay curves for batch adsorption systems incorporate three mass transport rate- controlling steps viz. external film mass transfer, diffusion in the adsorbent macro-pores and diffusion in the adsorbent micro-pores. The shape of the concentration decay curves with time varies according to the value of each of the above- mentioned three terms and their relative magnitudes[40].The effect of external mass transfer on adsorption kinetics can be investigated using the Mckay equation valid for negligible intra-particle diffusion.
 | (7) |
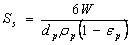 | (8) |
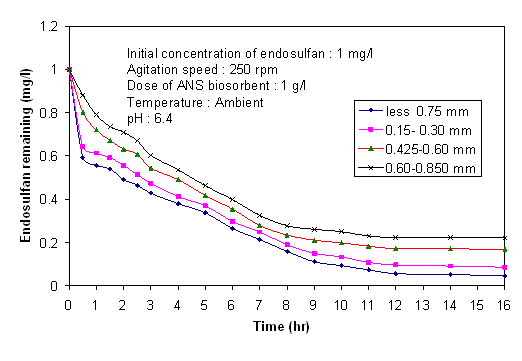
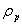 is the density, εp is the porosity (subscript p refers to particles).
is the density, εp is the porosity (subscript p refers to particles).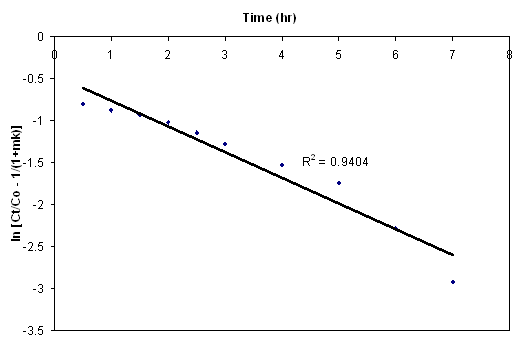 | Figure 5. Application of Mckay equation for analyzing effect of external mass transfer coefficient for endosulfan on ANS biosorbent |
3.4. Internal Diffusion Analysis
- Compared to the external or internal diffusion step, the adsorption step is usually very fast for the adsorption of organic compounds on porous adsorbents[42] and it is known that in the absence of internal diffusion, the adsorption equilibrium is reached within several minutes[43]. Thus, the long adsorption equilibrium time in present experiments suggests that the internal diffusion may be the rate-limiting step. To see this more clearly, an internal diffusion model based on Fick’s second law is used to treat the experimental data: where, qt is the amount of pesticide biosorbed on the fungal biomass at any time t(mg/g) and kid is the intraparticle diffusion rate constant(mg/g hr1/2). A plot of qt vs t1/2 should give a straight line if the adsorption is limited by the internal diffusion process[44].
3.4.1. Based on the Effect of Initial Adsorbate Concentration
- The effect of initial concentration also gives a general idea about rate limiting steps. Figure 6 show the amount of endosulfan biosorbed per g of ANS biosorbent versus t1/2 for initial concentration of 1 mg/L, 2 mg/L, and 4 mg/L for endosulfan. Initially in all the cases studied, a linear relationship between q versus t1/2 with a zero intercept was found, suggesting that the internal diffusion step dominated the biosorption process before the equilibrium was reached.
 | Figure 6. Intraparticle diffusion plots for endosulfan on ANS biosorbent at various initial concentrations |
3.4.2. Based on the Effect of Biosorbent Size
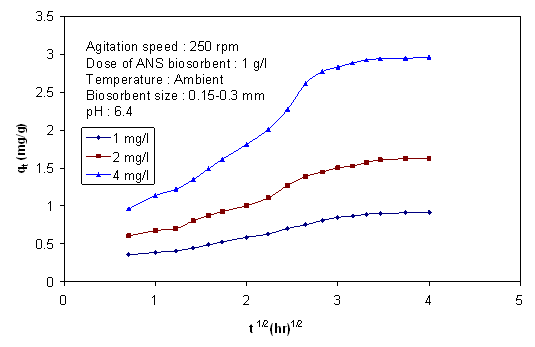
- It is possible to get an idea about rate limiting step from the change of pesticide concentration with time and biosorbent size. Figure 7 and 8 show the effect of biosorbent size on removal of endosulfan. As the size increased, percentage removal and removal rate decreased. The removal rates(k) obtained from the linear portion of the curves were(tabulated in Table 3) plotted against reciprocal of the adsorbent diameter as shown in Figure 9. According to Helfferich[34], for film diffusion to be rate limiting, the removal should linearly vary with reciprocal of the diameter of adsorbent. It is evident from graphs(Figure 8) that the film diffusion alone does not appear to play a significant role, on biosorption process in the case of biosorption of endosulfan on ANS biosorbent. The trend of curve indicates that the inclusion of both the external mass transfer diffusion and intra-particle diffusion may give more precise expression.
 | Figure 7. Effect of biosorbent size on change of concentration of endosulfan with time |
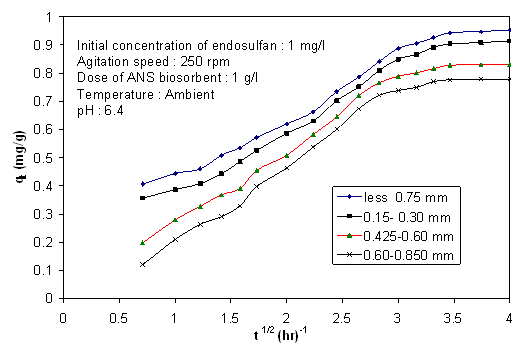 | Figure 8. Intraparticle diffusion plots for endosulfan on ANS biosorbent avarious biosorbent sizes |
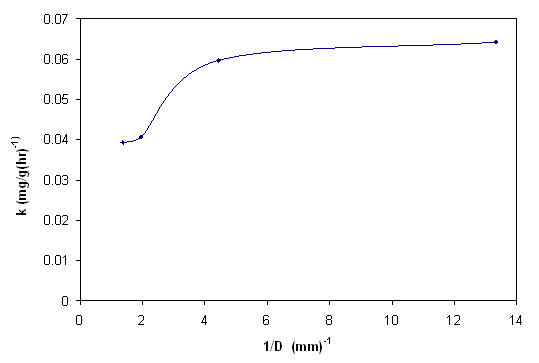 | Figure 9. Variation of rate constant with reciprocal of diameter of biosorbent |
|
3.4.3. Based on the Effect of Agitation
- The rate of agitation is one of the prominent factors for biosorption process as it helps keep adsorbent in suspension and gives proper mixing to solutes. Especially in the case of pesticides, which are hydrophobic in nature, agitation speed has a significant effect on the removal efficiency. Figure 10 depicts the influence of agitation speed from 175 to 250 on biosorption of endosulfan. The biosorption rate calculated from the agitation speeds of 175 rpm and 250 rpm were found to be 0.197 mg/g.hr1/2 and 0.22 mg/g.hr1/2 respectively. Both the curves had features like an initial curve portion, followed by a linear portion and later a plateau. Thus intra-particle diffusion along with film diffusion may be the controlling rate-limiting steps in the biosorption process.
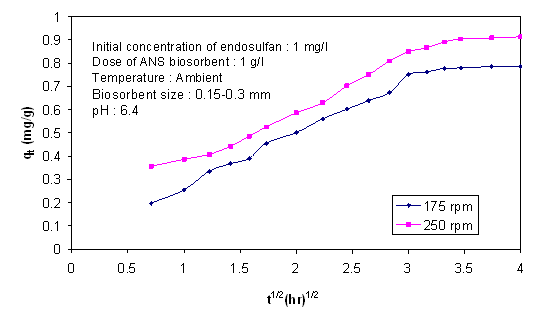 | Figure 10. Intraparticle diffusion plots for endosulfan on ANS biosorbent at various agitation speeds |
3.4.4. Based on Interruption Test
- Several factors of the adsorbents, adsorbate and solution phase are of importance in determining the rate-limiting step. The factors include the particle size of the adsorbent, concentration of adsorbate, degree of mixing, affinity of adsorbate for adsorbent and diffusion coefficients of the adsorbate in bulk solution and within the porous adsorbent. In an interruption test, the adsorbent is periodically removed from the adsorbate solution for a brief period and reimmeresed. When concentration gradients are present, the pause gives time for the gradients to level off within the pores of the adsorbent. When intraparticular transport is the rate limiting step, the rate of removal immediately after reimmersion is greater than the rate prior to interruption. However, the interruption period has no influence on the rate of biosorption after re-immersion when the external transport is rate limiting[34,22]. Figure 11 shows the effect of interruption test. A slight increase in the removal found after the interruption test indicated that intra-particle diffusion was rate limiting in biosorption process. From above all experimental results and discussion, it can be concluded that there are four steps involved in the entire biosorption process (1) migration of adsorbate molecules from bulk solution to the surface of the biosorbent; (2) diffusion through the boundary layer to the surface of the biosorbent; (3) biosorption at a site and (4) intraparticle diffusion into the interior of the biosorbent[45]. As temperature increased, the rate constant for intraparticle diffusion also increased, indicated that in the process of biosorption intraparticle diffusion played a significant role. From these observations, it can be concluded that diffusion needs some energy to overcome the mass transfer resistance. The results of other studies[45,46] also support this observation. The interruption test confirmed that intra-particle diffusion (pore diffusion) was important in determining the overall rate of the transfer of the adsorbate onto ANS biosorbent.
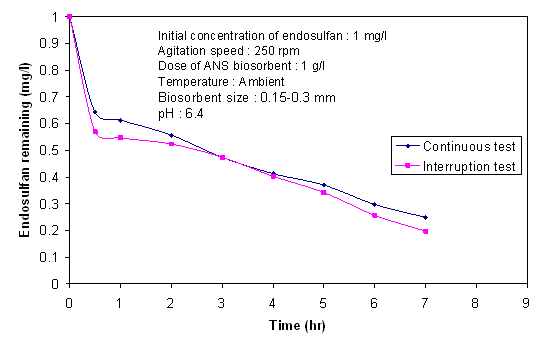 | Figure 11. Kinetic profiles of endosulfan biosorption onto ANS biosorbent interruption and un-interruption tests |
4. Conclusions
- In present study, sorption of endosulfan on ANS biosorbent was observed. The sorption capacity increased with an increase in initial endosulfan concentrations i.e 0.9636 mg/g, 1.6084 mg/g, 2.9413 mg/g for 1 mg/L, 2 mg/L and 4 mg/L respectively. The biosorption of endosulfan onto ANS biosorbent followed the pseudo-second-order kinetic model with high correlation coefficient sand also fitted well the intraparticle diffusion model upto 12 hrs. It is evident from Mckay equation that biosorption process on ANS biosorbent was mixture of different rate controlling steps. Plots of qt vs t1/2 indicated the effect of intra-particle diffusion on the process and also indicated that the pore diffusion alone may not be the rate controlling step. Interruption test confirmed that the pore diffusion played the most important role for the transfer of the adsorbate to the biosorption sites.
ACKNOWLEDGEMENTS
- The authors thank M/s Vijayalakshmi Insecticides, India, for providing technical grade of endosulfan for carrying out research outlined in the present research study.
References
| [1] | ATSDR, 2002, Toxicological profile of endosulfan prepared by the Agency for Toxic Substances and Disease Registry(ATSDR) [Online]. Available: http://www.atsdr.cdc.gov/toxprofiles/tp41.html |
| [2] | Sinha, N., Narayan, R., and Saxena, D.K., 1997, Effect of endosulfan on testis of growing rats., Bull. Environ. Contam. Toxicol., 58, 79–86 |
| [3] | Chaudhuri, K., Selvaraj, S., and Pal, A.K.,1999, Studies on the genotoxicology of endosulfan in bacterial system., Mutat. Res., 439, 63–67 |
| [4] | Sunderam,R.I.M.,. Cheng, D.M.H., and Thompson, G.B., 1992, Toxicity of endosulfan to native and introduced fish in Australia., Envi- Toxicol. Chem., 11, 1469–1476 |
| [5] | Paul, V., and Balasubramaniam, E., 1997, Effect of single and repeated administration of endosulfan on behaviour and its interaction with centrally acting drugs in experimental animals: A mini review. Toxicol. Pharmacol., 3, 151–157 |
| [6] | Sudhakar. Y, and Dikshit, A.K., 2001, Removal mechanism of endosulfan sorption on to wood charcoal., International J. Env. Poll., 15(5), 528-542 |
| [7] | Konstantinou, I.K., and Albanis, T.A., 2003, Photo catalytic transformation of pesticides in aqueous titanium dioxide suspensions using artificial and solar light: intermediates and degradation pathways., App. Cat. B: Env., 42, 319–335 |
| [8] | Gupta, V.K., Jain, C.K., Ali, I., Chandra, S., and Agarwala, S., 2002, Removal of lindane and malathion from wastewater using bagasse flyash from sugar industry waste., Water Research, 36, 2483–2490 |
| [9] | Kiso, Y., Sugiura, Y., Kitao T., and Nishimura, K., 2001, Effects of hydrophobicity and molecular size on rejection of aromatic pesticides with nanofiltration membranes., J. Membrane Sci., 92, 1-10 |
| [10] | Bollang, J.M., 1990. Metachlor herbicide pesticide degradation, soil decontamination, water decontamination, Comparison of Fusarium Sp., Mucor Racemous, Bacillus Circulans, Bacillus Megaterium, Unidentified Actinomycetes sp. with mixed culture, soil sample, Development of Indian Microbiology, 31,75-80 |
| [11] | Jury, W.A., Spencer, W.F., and Farmer, W.J., 1984, Behavior assessment model for trace organics in soil: iii. application of screening model., J. Environ. Qua., 13, 573-579. |
| [12] | Wong, P.K. and Kwok, S.C., 1992, Accumulation of nickel ion(Ni2+) by immobilized cells of Enterobacterspecies., Biotechnol. Lett., 14(7), 629-634 |
| [13] | Modak, J.M., Natarajan, K.A., 1995, Biosorption of metals using nonliving biomass - A review. Minerals and Metallurgical Processing, 95, 189-196 |
| [14] | Volesky, B., and Holan, Z.R., 1995, Biosorption of heavy metals. Biotechnol Prog.,11, 235–50 |
| [15] | Veglio, F., and Beolchini, F., 1997, Removal of metals by biosorption: A review. Hydrometalurgy, 44, 301–16 |
| [16] | Aksu, Z., and Tezer, S., 2000. Equilibrium and kinetic modelling of biosorption of Rremazol Black B by R. arrhizus in a batch system: Effect of Temperature. Process Biochem, 36, 431-439 |
| [17] | Bell, J. P., and Tsezos, M., 1987, Removal of hazardous organic pollutants by adsorption on microbial biomass. Water Sci Technol, 19, 409-416 |
| [18] | Benoit, P., Barriuso, E., and Calvet, R., 1998, Biosorption characterization of herbicides, 2, 4-D and Atrazine and 2 Chlorophenols on fungal mycelium., Chemosphere, 37, 1271–1282 |
| [19] | O’Mahony, T., Guibal, E., and Tobin, J. M., 2002, Reactive dye biosorption by Rhizopus arrhizus biomass Enzyme Microbial Technol., 31, 456–463 |
| [20] | Juhasz, A.L., Smith, E., Smith, J., and Naidu, R., 2002, Biosorption of organochlorine pesticides using fungal biomass., J. Ind. Micro. . Biotech., 29, 163 – 169 |
| [21] | Rao, J.R., and Viraraghavan, T., 2002, Biosorption of phenol from an aqueous solution by Aspergillus niger biomass., Bioresour. Technol., 85, 165–71 |
| [22] | Zogorski, J.S., Faust, S.D., and Jr.Hass, J.H., 1976, The kinetics of adsorption of phenol by granular activated carbon, J. Colloid Interface Sci., 55(2), 329-341 |
| [23] | Aly, O.M., and Faust, S.D., 1965, Removal of 2, 4-dichlorophenoxy acetic acid derivatives from natural waters, J. AWWA, 221-230 |
| [24] | Tsezos, M. and Wang, X. 1991, Study on the kinetics of hazardous pollutants adsorption and desorption by bio-mass: mechanistic consideration, J. of Chem. Tech. Biotech., 50, 507-521 |
| [25] | Khoo, K. M., and Ting, Y. P., 2001, Biosorption of gold by immobilized fungal biomass, Biochemical Eng. J., 8, 51–59 |
| [26] | Tsai, W., and Lai, C., 2006, Adsorption of herbicide paraquat by clay mineral regenerated from spent bleaching earth” J. Haz. Mat., B134, 144–148 |
| [27] | Namasivayam, C., and Sumithra, S., 2005, Removal of direct red 12b and methylene blue from water by adsorption onto fe(iii)/cr(iii) hydroxide, an industrial solid waste, J. Envi. Man., 74, 207–215 |
| [28] | Parab, H., Joshi, S., Shenoy, N., Verma, R., Lali, A., and Sudersanan, M., 2005, Uranium removal from aqueous solution by coir pith: equilibrium and kinetic studies., Tech., 96, 1241–1248 |
| [29] | Aksu, Z., 2001, Biosorption of reactive dyes by dried activated sludge: equilibrium and kinetic modeling., Biochem. Eng. J., 7, 79–84 |
| [30] | Allen, S.J., Gan., Q., Matthews, R., and Johnson, P.A., 2005, Kinetic modeling of the adsorption of basic dyes by kudzu, J. Colloid Interface Sci., 286, 101–109 |
| [31] | McKay, G., YS, H., and JCY, N., 1999, Biosorption of copper from waste waters: A review. Sep Pur Methods, 28, 87–125 |
| [32] | Karaca, S., Gurses, A., Ejder, M., and Ackyldz, M., 2004, Kinetic modeling of liquid-phase adsorption of phosphate on dolomite, J. Colloid Interf. Sci., 277, 257–263 |
| [33] | You, L., Wu, Z., Kim, T., and Lee, K., 2006, Kinetics and thermodynamics of bromophenol blue adsorption by a mesoporous hybrid gel derived from tetraethoxysilane and bis(trimethoxysilyl) hexane J. Colloid Interf. Sci., 300, 526–535 |
| [34] | Helfferich, F., 1962, Ion exchange, McGraw Hill, N. W., bpp. 72-94 |
| [35] | Michaels, A. S., 1952, Simplified method of interpreting kinetics data in fluid-bed ion exchange, Industrial Engg. Chem., 44, 1922 |
| [36] | Wu, S.C., and Gschwend, P.M., 1986, Sorption kinetics of hydrophobic organic compounds to natural sediments and soils, Environ. Sci. Tech., 20, 717-725 |
| [37] | Conolly, J. P.1980. Ph. D. Dissertation, University of Texas at Austin |
| [38] | Weber, W.J.(Jr.) and Digiano, F. A. 1996, Process dynamics in environmental system, John Wiley and Sons Inc., NY |
| [39] | Chang, C.Y., Tsai, W.T., Ing, C.H., and Chang, C.F., 2003, “Adsorption of polyethylene glycol(PEG) from aqueous solution on to hydrophobic zeolite, J. Colloid Interf. Sci., 260, 273–279 |
| [40] | Mckay, G. S., Mckee, S., and Walters, H. R. J., 1987, Solid-liquid adsorption based on the external mass transfer, micro-pore and micro-pore diffusion, Chem. Egg. Sci., 42(5), 1145-1151 |
| [41] | Dutta,M., Baruah, R., and Dutta, N.N., 1997, Adsorption of 6-aminopenicillanic acid on activated carbon, Seperation and Purification Ttech., 12, 97-108 |
| [42] | Sarkar, M., Acharya, P., and Bhattacharya, B., 2003. Modeling the adsorption kinetics of some priority organic pollutants in water from diffusion and activation energy parameters J. Colloid Interf. Sci., 266, 28–32 |
| [43] | Mak, S., and Chen, D., 2004, Fast adsorption of methylene blue on polyacrylic acid-bound iron oxide magnetic nano particles, Dyes and Pigments, 61, 93–98 |
| [44] | Bhattacharyya, K.G, and Sharma, A., 2004, Azadirachta indica leaf powder as an effective biosorbent for dyes:a case study with aqueous congo red solutions., J. Env. Man., 71, 217–229 |
| [45] | Wu, J. and Yu, H., 2007, Biosorption of 2, 4-Dichlorophenol by immobilized white-rot fungus hanerochaete chrysosporium from aqueous solutions, Bioresource Tech., 98, 253–259 |
| [46] | Namasivayam, C. and Ranganathan, K., 1995, Removal of Cd(II) from wastewater by adsorption on Waste Fe(III)/Cr(III) hydroxide, Water Research, 29(7), 1737–1744 |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML