-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Microbiology Research
p-ISSN: 2166-5885 e-ISSN: 2166-5931
2011; 1(1): 5-7
doi: 10.5923/j.microbiology.20110101.02
Antibacterial Activities of Asmina triloba against Some Bacterial Pathogens
Abalaka M. E , Oyewole O. A.
Department of Microbiology, Federal University of Technology, PMB 65, Minna, Niger State, Nigeria
Correspondence to: Oyewole O. A. , Department of Microbiology, Federal University of Technology, PMB 65, Minna, Niger State, Nigeria.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
The antibacterial effect of Asmina triloba against Pseudomonas aeruginosa, Klebsiella ozanae, Staphylococcus aureus and Escherichia coli was determined using the agar cup plate technique. The phytochemical components of Asmina triloba showed the presence of alkaloids and phlobatanin and the absence of saponin, tannins, phenolics, glycosides, flavonoids and triterpenes. The results showed that the test organisms were susceptible to 500mg/ml, 50mg/ml and 5mg/ml of the plant extract. The minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) were determined. The result showed that the MIC for Pseudomonas aeruginosa and Klebsiella ozanae was 500mg/l and the MIC of 50mg/l was recorded for S. aureus and E.coli. No MBC was recorded for both P. aeruginosa and K. ozanae but MBC for S. aureus and E. coli was 500mg/l. The results of the study suggest that extracts of Asmina triloba could be suitable for the treatment of various infections caused by P. aeruginosa, K. ozanae, S. aureus and E. coli.
Keywords: Antibacterial Effect, Asmina triloba, Phytochemical Components, Minimum Inhibitory Concentration, Minimum Bactericidal Concentration
Cite this paper: Abalaka M. E , Oyewole O. A. , "Antibacterial Activities of Asmina triloba against Some Bacterial Pathogens", Journal of Microbiology Research, Vol. 1 No. 1, 2011, pp. 5-7. doi: 10.5923/j.microbiology.20110101.02.
1. Introduction
- The term herbal drug determines the part/parts of a plant used for preparing herbal and traditional medicines (for examples: leaves, flowers, seeds, roots, barks, stems, etc.) (Kayode and Kayode, 2011) Furthermore, World Health Organization, WHO (2001) defines medicinal plant as herbal preparations produced by subjecting plant materials to extraction, fractionation, purification, concentration or other physical or biological processes which may be produced for immediate consumption or as a basis for herbal products. Medicinal plants contain biologically active chemical substances such as saponins, tannins, essential oils, flavonoids, alkaloids and other chemical compounds (Sofowora, 1996) which have curative properties. These complex chemical substances of different compositions are found as secondary plant metabolites in one or more of these plants. Tyler (1999) has reported that plants also contain certain other compounds that moderate the effects of the active ingredients.North American pawpaw (Asmina trilobota) is the largest tree fruit native to temperate North America. It is an underutilized plant that has potential as landscape tree, fruit crop and as a source of pharmaceutical products (Finneseth et al., 2000). In addition, A. triloba has some identified secondary products (acetoginins) in the bark and leaves that have a wide range of biological activities including anticancer, antimicrobial, immune suppressant and pesticidal properties (Finneseth et al., 2000).This study was undertaken therefore, to determine the phytochemical components of the leaf extracts of A. triloba, the minimum inhibitory concentration (MIC) of the extract on Pseudomonas aeruginosa, Klebsiella ozanae, Staphylococcus aureus and Escherichia coli, the minimum bactericidal concentration (MBC) of the extract on Pseudomonas aeruginosa, Klebsiella ozanae, Staphylococcus aureus and Escherichia coli.
2. Materials and Methods
- Collection and Preparation of SamplesThe bark of A. triloba was collected from different locations in Minna metropolis. It was air-dried for six weeks in microbiology laboratory of Federal University of Technology, Minna. The dried materials were pulverised in mortar and packaged in bottles for analysis. Collection of SpecimenPure cultures of Pseudomonas aeruginosa, Klebsiella ozanae, Staphylococcus aureus and Escherichia coli were obtained from General Hospital Minna. Niger State and were subcultured in agar slants.Extraction of MaterialsEthanol and water were used as solvents for the extraction of the plant materials. 150g of pulverised sample was suspended in 750ml of 75% ethanol for 120hours. The extracts were decanted, filtered and evaporated in vacuole at 450C.Phytochemical Screening of Extracts of Asmina triloba The phytochemical components of extracts of A. triloba was determined using methods described by Odebiyi and Sofowora (1978) and Trease and Evans (1989). The phytochemical components analysed for were alkaloids, tannins, phenolics, glycosides. saponins, flavonoids, steroids, phlobatanins and triterpenes.Antimicrobial Susceptibility TestSusceptibility test of the test organisms to extracts of A. triloba at concentrations of 500mg/ml, 50mg/ml and 5mg/ml was carried out using agar cup plate technique as described by Silver et al. (1997). Nutrient agar was prepared using autoclave at 121℃ for 15 minutes. It was then poured on to plates and allowed to solidify. Standardized inoculum of each test organisms was spread on to agar plates so as to achieve a confluent growth. The impregnated discs with different concentration of the extract were placed on the surface of the medium at three points equidistant from one another. The plates were then incubated at 37℃ for 24 hours.Determination of Minimum Inhibitory Concentration (MIC)The minimum inhibitory concentration (MIC) of the test organisms was determined using the tube dilution technique. 9ml of the nutrient broth was pippeted into various test tubes containing concentrations of 500 mg/ml, 50 mg/ml and 5 mg/ml of the extract. The overnight culture of the test organisms diluted at 106cfu/ml was added to the test tubes and then incubated at 37℃ for 24 hours. The least concentration of the extract that did not indicate any visible growth of the incubated organisms in broth culture was taken as the minimum inhibitory concentration (MIC) (Hugo and Russel, 1983).
3. Results
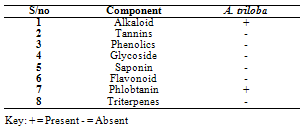
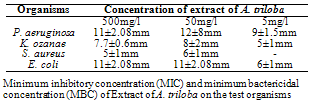
- Phytochemical Screening of the ExtractsTable 1 shows the phytochemical screening of the extract of A. triloba. The phytochemical components of A. triloba showed the presence of alkaloid and phlobatanin but the absence of tannins, phenolics, glycosides, saponin, flavonoids and triterpenes.Antimicrobial Activities of the ExtractsTable 2 shows the zones of inhibition (mm) of extract of A. triloba at different concentrations (mg/ml). At 500mg/ml, P. aeruginosa and E. coli had a zone of inhibition of 11 ± 2.08mm and S. aureus had the least zone of inhibition of 5 ± 1mm. At 50 mg/ml, P. aeruginosa had the highest zone of inhibition of 12 ± 8mm and the least zone of inhibition of 6 ± 1mm was obtained by S. aureus. At 5mg/ml, S. aureus was not sensitive to extract of A. triloba but P. aeruginosa had the highest zone of inhibition of 9 ± 1.5mm.
|
|
|
|
|
4. Discussion
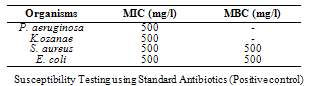
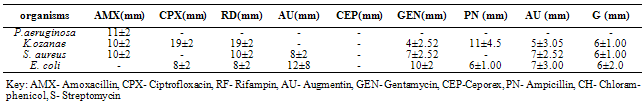
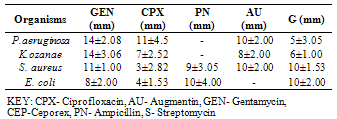
- The phytochemical components of A. triloba (Table 1) showed the presence of alkaloid and phlobatanin. The presence of these components may be responsible for the antibacterial effects of the bark extract of A. triloba. Avalos et al. (1993) reported that alkaloid have a drastic lethal effect on the central nervous system while phlobatanin have protective ability against bacterial and fungal infections. Sofowora (1996) reported that phytochemical components usually interfere with growth and metabolisms of microorganisms. Oderinde et al. (2002) reported that A. triloba is use tropically in the treatment of cuts, rashes, stings and burns.A. triloba shows minimum inhibitory concentration (MIC) value of 500mg/ml for P. aeruginosa, K. ozanae, S. aureus and E. coli. MBC values for P. aeruginosa and K. ozanae was 500mg/ml whereas Staphylococcus aureus and E. coli had no MBC value (Table 3). This suggests that the bark extracts of A. triloba is bacteriostatic on the tested organisms. According to Prescott et al. (2005), a bacteriostatic agent kills at a much higher concentration whereas drug kills pathogen at levels only two or four times the MIC.At 500mg/ml and 50mg/ml and 5mg/ml, the extracts of A. triloba showed a higher susceptibility on P. aeruginosa and E. coli. The least zone of inhibition was recorded in S. aureus while at 5 mg/ml, S. aureus was resistant (Table 2). When compared with standard antibiotics (Table 4), extracts of A. triloba had a higher zone of inhibition on E. coli. This may indicate that extracts of A. triloba has a higher antibacterial effects on gram negative E. coli than the tested antibiotics.The result of this study shows that at high concentration, bark extract of A. triloba has antibacterial effects against P. aeruginosa, K.ozanae, S. aureus and E. coli.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML