-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Basic Sciences of Medicine
p-ISSN: 2167-7344 e-ISSN: 2167-7352
2021; 10(1): 7-9
doi:10.5923/j.medicine.20211001.02
Received: Apr. 22, 2021; Accepted: May 24, 2021; Published: Jun. 15, 2021

Verification of Sacralisation and Simultaneous Occurrence of Congenital Anomalies
1Department of Anatomy, UPUMS Saifai Etawah, UP, India
2Department of Orthopedics, UPUMS Saifai Etawah, UP, India
Correspondence to: Rajani Singh, Department of Anatomy, UPUMS Saifai Etawah, UP, India.
| Email: |  |
Copyright © 2021 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Very common disease, backpain is often debilitating/disturbing day to day activities of patients. One of the causes of backpain is sacralisation. Sacralisation is caused by congenital factors besides, others as various degrees of Sacralisation and other associated anomalies, like supernumerary digits, cleft palate, absence of teeth, disorders of thymus and parathyroid, are caused by mutation of HOX 11 and deficiency of Pax1/Pax9 paralogous genes in mice. HOX 11 and Pax1/Pax9 are also present in human beings so natural mutation can also occur in them. Therefore, as per hypothesis, the varied degree of sacralisation associated with aforementioned anomalies may be present in human being also. So, if backpain associated with sacralisation is detected during diagnosis of backpain by clinicians, other anomalies can be verified to establish the hypothesis. Therefore, a project has been designed to carry out this study. Aim of the study was to establish this hypothesis. For this, 400 cases of backpain in the age group of 25-50 were observed. Out of 400 cases, complete sacralisation was observed in 4 patients and these were not found associated with other congenital anomalies as observed in mice. This may be due to process of redundancy of genes. But definite conclusion can only be derived after adding the results of genetic studies.
Keywords: Back pain, Sacralisation, Cleft palate, Pax1/Pax9 genes
Cite this paper: Rajani Singh, SPS Gill, Verification of Sacralisation and Simultaneous Occurrence of Congenital Anomalies, Basic Sciences of Medicine , Vol. 10 No. 1, 2021, pp. 7-9. doi: 10.5923/j.medicine.20211001.02.
Article Outline
1. Introduction
- The backpain is very common cause of morbidity affecting day to day activities among the people. Numerous causes, in medical examination, have been attributed to low back pain (LBP). A long list exists but the enlistment of sacralization, as one of the causes, has resulted in a lot of controversy. Numerous studies have reported the presence of this anomaly in a back pain population [1,2] such as Castellvi et al. [3] reported a 30% prevalence of sacralisation in low backpain patients and Otani et al. [4] have reported the 13% incidence of sacralization in patients with LBP. But several investigators have the opinion that sacralization is incidentally diagnosed and has no clinical impact [5,6]. However, this alteration may contribute to incorrect identification of a vertebral segment. But the most pertinent question is ‘what is sacralisation?’ Sacralisation is fusion of 5th lumbar vertebra to the 1st sacral vertebra. Normally, the sacralization is a congenital vertebral anomaly of the lumbosacral spine [3]. It may also be due to arthritic changes through aging in elderly population. But congenitally, the effect of mutant PAX1/9 genes’ expression cause several types of sacralisation depending on degree of mutation in mice [7]. Further, the experiments on mice, that were deficient in Pax1/ Pax9 paralogous genes in varying degrees, has also revealed several malformations such as fused vertebrae, mainly involving the lumbar and caudal regions, split vertebrae as well as ossified fusion between vertebrae along with neural arches [8]. It has also been observed in mice that deletion of Hox11 from mice influence patterning so resulted in formation of lumbar vertebrae even below L5 in sacral region [9]. Thus, sacralisation may be caused by deficient in Pax1/ Pax9 paralogous genes in mice together with presence of mutant Hox 11 gene patterns the sacral vertebrae and their over expression is expected to produce signs of sacralization or caudalization at other levels of the axial skeleton [8,9]. During mouse embryogenesis, the Pax1 and Pax9 genes are expressed in the 3rd and 4th pharyngeal pouches, the ultimobranchial body and in developing limbs in mice [10]. Hence, Pax1/Pax9 genes are essential for the organogenesis of thymus, the parathyroid, the ultimobranchial body and the limbs of mice. Mice homozygous for Pax1/Pax9 deletion lack a thymus, a parathyroid, and ultimobranchial bodies. Teeth are also absent. The secondary palate is cleft. Supernumerary digits are formed and the flexor of hind limb is missing [11]. These two sets of genes are also seen in other vertebrate including human beings and same functions of these genes are expected in human beings also similar to those in mice. Based on these findings hypotheses was formulated by Singh (2012) [7]. According to Singh 2012 sacralisation may be caused by varied expression of Pax1/Pax9 and this anomaly may be associated with lack a thymus, a parathyroid, absence of teeth, presence of cleft secondary palate and Supernumerary digits in human beings also like mice. Thus, aim of the present study is to verify this hypothesis. If this hypothesis is verified, patients with sacralisation can be simultaneously screened and treated for associated congenital anomalies. Therefore, the study was carried out.
2. Material and Methods
- The studywas carried out in the department of Anatomy in collaboration with Orthopaedics department of UPUMS Saifai Etawah UP India. The patients were examined in OPD of department of Orthopaedics. Patients in the age group of 25-50 years, complaining backpain were screened for sacralisation by X-ray revealing complete fusion of 5th lumbar and 1st sacral verterbra, split vertebrae, absence of neural arches, absence of transverse process as well as ossified fusion between vertebrae and neural arches. Frontal (AP) and lateral lumbosacral regions were evaluated in these X-rays. The radiographs were examined, and data was collected and analysed. If sacralisation was detected in X-ray of these patients, these patients were examined for secondary cleft palate. Supernumerary digits, deformities of teeth. For underdevelopment of thymus, patients were asked if they suffered from repeated infections in childhood and for parathyroid, blood level of parathyroid hormone was measured. All the patients involved in the study were informed that their data will be used in research without disclosing their identity. The ethical clearance from institutional ethical committee was taken vide no. 63/2020-21. Elderly patients above the age 50 and patients below 25 years were excluded from the study as back pain and sacralisation is common in elderly people due to arthritic changes.
3. Results
- 400 patients in the age group 25-45 years with back pain were examined for sacralisation. Complete Sacralisation (Figure 1) was observed in 4 patients with backpain constituting 1% while split vertebrae, and absent neural arches, ossified fusions between vertebrae and neural arches, absence of transverse processes and pedicles were not detected in any of these patients with backpain.
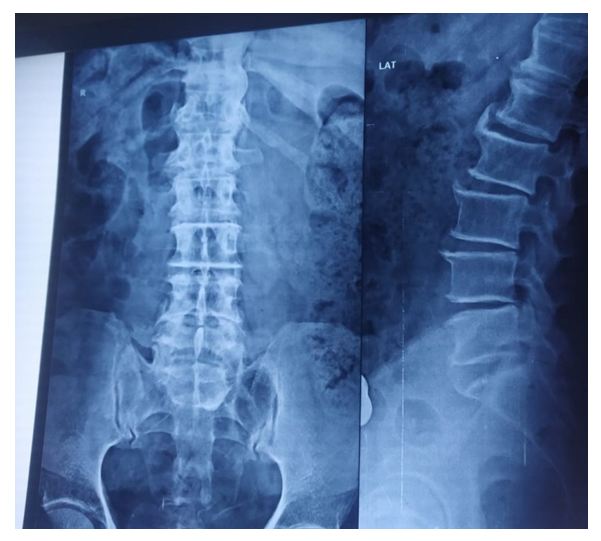 | Figure 1. Showing complete sacralsation in X-ray of lumbosacral region |
4. Discussion
- The Hox11 group is important for the formation of the sacral and caudal vertebrae [9] and thus their over expression is expected to produce signs of sacralization or caudalization at other levels of the axial skeleton. The patterning by the Hox11 group requires a combination of instructions given in the segmental plate and later in the somite. The formation of sacral structures is due to expression of Hox group 11 genes in the presomitic mesoderm and caudal vertebrae are formed due to activity of these genes in the somite. But both areas are affected when all six Hox group 11 alleles are inactivated [9]. In mice that are deficient for one functional copy of Pax1, heterozygosity and homozygosity of Pax9 mutations result in vertebral malformations such as fused vertebra, split vertebra, and ossified fusions between the vertebrae and neural arches [8] (Peters et al., 1999). Moreover, in mutant mice, a reduction in or the absence of transverse process and pedicles are also seen in caudal vertebra [12]. These anomalies are most commonly observed in the lumbar and caudal region and might be either due to decreased cell proliferation or increased cell death since the Pax1/Pax9 genes are required to maintain high rates of cell proliferation during sclerotome development. Further, an increased apoptosis has been observed in Pax1/ Pax9 deficient mice. These processes are gene dosage-dependent [8]. During mouse embryogenesis, Pax1/Pax9 are also expressed in the 3rd and 4th pharyngeal pouches, the ultimobranchial body and the developing limbs [10]. Hence the Pax1/Pax9 genes are essential for the formation of the thymus, parathyroid, ultimobranchial body and limbs of mice. Mice homozygous for Pax1/Pax9 deletion lack a thymus, parathyroid, and ultimobranchial bodies. Teeth are also absent. The secondary palate is cleft. Supernumerary digits are formed and the flexor of hind limb is missing [11]. Besides producing sacralisation/defective sacralisation or caudalisation/ defective caudalisation in human beings analogous to those seen in mice, a modulated effect of the actions of Hox11 and Pax1/9 mutant genes may generate above mentioned anomalies in human beings too simultaneously [7]. But in present study, in patients with sacralisation, associated anomalies were not observed as advocated by Singh [7] (2012). This may be due to process of redundancy of HOX 11, Pax1/Pax9 genes.
5. Conclusions
- In current study, out of 400 hundred pateints of backpain, incidence of sacralisation was observed in 4 patients constituting 1% of the population. Associated anomalies; lack a thymus, parathyroid, absence of teeth, presence of secondary cleft palate and supernumerary digits were not observed in any of the case which may be due redundance of HOX11, Pax1/Pax 9 genes during evolution. Further genetic studies are recommended in the patients of sacralisation to confirm whether HOX 11, Pax1/Pax9 genes are also expressed in sacrum, 3rd and 4th pharyngeal pouches and the developing limbs, thymus, parathyroid during their genesis in human beings also to fully establish the hypotheses formulated by Singh (2012).
Disclosure
- This project is funded by UPUMS Saifai Etawah Govt. of UP India.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML