Rajani Singh
Department of Anatomy, Uttar Pradesh University of Medical Sciences Saifai, Etawah, India
Correspondence to: Rajani Singh, Department of Anatomy, Uttar Pradesh University of Medical Sciences Saifai, Etawah, India.
| Email: |  |
Copyright © 2021 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
Spinal cord is part of central nervous system enshrined in upper 2/3rd of vertebral canal. It contains various ascending tracts which carry sensory information to the brain and descending tracts brings motor commands from brain so that proper action is executed. Aim of the this review is to consolidate morbid anatomy and compare with the normal anatomy of spinal cord which will provide ready reference to neurosurgeons while carrying out various neurosurgical interventions. The literature was explored using the terms related to normal and morbid anatomy of spinal cord using the databases; Sceilo. Pubmed, Medline, research gate etc. Only English language article were taken into account. Some standard text books of Anatomy like Gray’s Anatomy, Cunningham’s manual, Snell’s neuroanatomy etc. were also consulted. Literature survey brought out various syndromes like anterior cord syndrome, posterior cord syndrome, central cord syndrome, conus medullaris syndrome. These syndromes cause various neurological signs and symptoms due to distorted anatomy. The knowledge and information enshrined in this work will of be of utmost use to neurosurgeons in carrying out diagnosis and neurosurgical procedures successfully, with least failure rate.
Keywords:
Spinal cord, Morbid anatomy, Conus medullaris, Spinal cord syndrome
Cite this paper: Rajani Singh, Morbid Anatomy of Spinal Cord - A Review, Basic Sciences of Medicine , Vol. 10 No. 1, 2021, pp. 1-6. doi: 10.5923/j.medicine.20211001.01.
1. Introduction
The normal Anatomy of the Spinal Cord:The spinal cord is elongated narrow, cylindrical structure constituted by nervous tissue. It starts from the medulla oblongata in the brainstem extending to the bottom of L1 vertebra where it forms the conus medullaris. The spinal cord is located inside the vertebral canal which is formed by the vertebral foramina of 7 cervical, 12 thoracic, 5 lumbar, and 5 sacral vertebrae. The spinal cord is composed of 31 segments, namely 8 cervical, 12 thoracic, 5 lumbar, 5 sacral and 1 coccygeal segments. This cord composed of white and gray matter. The grey matter is enveloped by white matter. The white matter at the cord’s periphery contains ascending and descending tracts or neural pathways of myelinated sensory and motor nerve fibers. These tracts are organized somatotopically within the spinal cord [1]. In transverse sections (Fig. 1), the gray matter is arranged in butterfly shaped (H-shaped) composed of cell bodies and non-myelinated fibers and divided into dorsal (posterior), ventral and lateral horns. 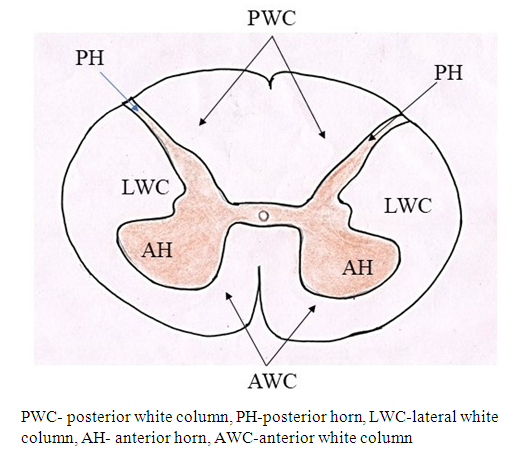 | Figure 1. Internal structure of spinal cord in transverse section |
The dorsal horns contain sensory fibers that originate from neurons in this horn. The gray matter contains many internuncial and motor neurons; those carry motor, sensory, or reflex impulses, from dorsal to ventral nerve roots, from one side of the cord to the other, or from one level of the cord to another. The neurons of the dorsal horns collect ascending sensory information fed to the spinal cord through dorsal roots of the spinal nerves. The lateral horns are situated basically in the thoracic region and contain the preganglionic visceral motor neurons that project to the sympathetic ganglia. Ventral horns contain lower motor neurons, which receive impulses from the motor cortex via the descending corticospinal tracts and at the local level from internuncial neurons and afferent fibers from muscle spindles. The ventral roots of the spinal nerves, formed of axons arising from motor neurons located in ventral horns, innervate striated muscles. The sensory, motor and autonomic tracts related to specific functions constitute dorsal, ventral and lateral, columns of white matter of the spinal cord. The dorsal columns carry ascending sensory information from somatic mechanoreceptors. The lateral columns include axons that travel from the cerebral cortex to contact spinal motor neurons. These pathways are also referred to the cortico-spinal tracts. The ventral constituted by ventrolateral or anterolateral columns carry both ascending information about pain and temperature and descending motor information. Some general rules of spinal cord organization are (1) that neurons and axons that process and relay sensory information are found dorsally; (2) that preganglionic visceral motor neurons are found in an intermediate/lateral region; and (3) that somatic motor neurons and axons are found in the ventral portion of the cord [2]. Various neural tracts are shown in Figure 2.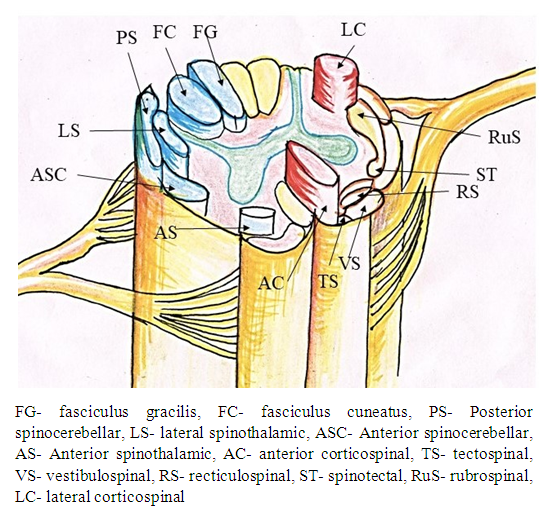 | Figure 2. Showing main tracts of spinal cord |
Normal vascular supply to spinal cord:The blood supply to spinal cord takes place through extrinsic and intrinsic network of arterial systems. The intrinsic arterial supply to the spinal cord consists of two arterial systems (Fig. 3) namely 1. One anterior spinal artery and two posterior spinal arteries irrigating anterior and posterior regions of spinal cord [3,4,5,6]. 2. Lumbar and posterior intercostal arteries, the subclavian arteries, the hypogastric arteries, and vasculature of the paraspinous muscles [4,5,7] forming collateral intrinsic arterial system.Thus in case of disruption of blood irrigation from a single segmental artery, the collateral arterial supply takes care of spinal cord. The area of spinal cord, irrigated by an artery, having insufficient collateral supply, may be effected with partial/full ischemia in case of obstruction of blood flow by the artery [4,7,8,9]. The posterior region of spinal cord is very rarely affected by neurologic ischemia [3,4,5,6] because it is irrigated by sufficient blood flow through collateral arterial network from the dual posterior spinal arteries. On the other hand, the anterior region of spinal cord is supplied by anterior spinal artery having insufficient collateral blood supply so it is prone to ischemic disease [4,7]. 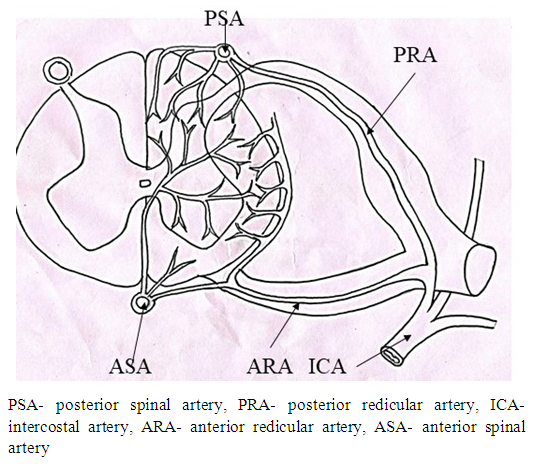 | Figure 3. Showing arterial supply of spinal cord |
What is morbid Anatomy?Spinal cord disorders (SCD) produced by spinal cord injuries (both intrinsic and extrinsic) alter normal Anatomy of Spinal Cord. This modified anatomy is termed as morbid Anatomy (MA) of spinal cord. All the structures described in internal anatomy of spinal cord are widely different from one another from view point of degree of softness/hardness. So these modifications/distortions are produced by external trauma or internal injury by compression of softer structures by harder structures. So there is very intimate relationship between morbid Anatomy and SCD if the relationship is standardized and calibrated through one to one correlation between SCD and MA. MA can be detected by imagery with the help of anatomical knowledge. As structural configuration of network of nerve fibers pertaining to peripheral system of spinal cord is very complex forming the two way highway of information flow to and fro to brain and peripheral structures producing signs and symptoms due to SCD. As such neurosurgical interventions, to treat morbid structures due to spinal cord disorders/injury in accessing and manipulating complex configuration of unknown and unidentified macro/microneural network (anatomical structures) is always at risk to be damaged, are extremely dangerous so to comprehend configuration of normal/variant anatomical structures for diagnosing and treating morbidity are of paramount importance for safe procedures. Therefore, very often postoperative iatrogenic lapses and /or invasions in spinal cord has been the order of the day. This is why; a review study of morbid Anatomy of spinal cord consequent upon SCD has been aimed at producing a record of morbid Anatomy corresponding to various SCD to facilitate the neurosurgical procedures.
2. Material and Methods
The study will encompass precise knowledge of morbid Anatomy of spinal cord in relation to normal Anatomy to identify the distortions in the morbid spinal cord structures to facilitate accurate imagery interpretation corresponding to morbidity together with precise identification of surrounding anatomical variant/normal structures, pathways and their configuration to save the invasive damage during neurosurgical access and intervention through review of literatures. In addition to this, the detailed knowledge of SCDs, their causative and varying imprints/signatures during the disease process at macro/micro level will be analyzed to improve the success rate in risky neurosurgical intervention and imagery interpretation. The study was carried out through review of literature supported by analysis of disorders causing distortions in normal Anatomy of spinal cord through knowledge, experience, skill and expertise of the authors. The study will consolidate the case wise knowledge pieces into one place for ready reference to neurosurgeons. The literature was surveyed for neurovascular structures in general and multitude of syndromes in particular using the terms, ‘spinal cord syndromes’, ‘clinical complications in spinal cord due to compression by extra-growth in bones or any harder structures, spinal cord disorders and corresponding morbid Anatomy’, anterior cord syndrome, posterior cord syndrome, central cord syndrome, cauda equine syndrome, conus medullaris syndrome spinal cord disorders, split spinal cord and spina bifida. Search was done using data bases: pubmed, researchgate, willeys on line library, medline, Scielo. Only English language article and standard text books were referred. Search was conducted during March 2020 to Oct. 2020.
3. Results and Discussion
Spinal cord disease/morbidity results from multiple diverse pathologic processes. Trauma is the most common cause of spinal cord injury. Depending on its pathogenesis, spinal cord disease is manifested with variable impairment of motor, sensory, and/or autonomic function due to distortions in structures, organs, limbs and systems producing signs and symptoms of discomfort due to these impairments. This review focuses on morbid anatomy of spinal cord corresponding to diseases/disorders. Various spinal cord disorders are grouped into spinal cord syndromes apart from other macro/microstructural injuries which are discussed and elaborated below.Relation between spinal cord disorders and morbid anatomy: The spinal cord is universal bidirectional highway of information flow from body to brain and vice-versa. All activities of human body are monitored/controlled by brain through its cognitive functions via information system. The sensory tracts carry the sensory information from skin, structures, organs, limbs and systems via dorsal root ganglions, dorsal roots and dorsal column to the brain which, in response, issues the commanding instruction for protection and maintenance via motor tracts through motor nerve roots back to muscles of the body. Thus sensory bus is plying loaded with sensory input from body or environment to unload the sensory information in the brain and, in response, the brain loading the commands on motor bus for activity and/or counter the threat is passed on to body muscles. Thus SCDs/syndromes, expressed by signs and symptoms, are generated by extrinsic/intrinsic injury to tracts, neurons and axons of spinal cord interrupting the traffic of bidirectional information.The signs and symptoms of SCDs/syndromes are generated by interruption of information flow due to injured structures, organs, limbs and systems distorting them leading to impairment of functions and/or activities of these structures, Thus SCDs/syndromes are always associated to each other so SCDs/syndromes can be identified by corresponding morbid Anatomy of spinal cord.Spinal Cord Syndromes and associated morbid anatomy:Neuro-morbidity:Injury to the dorsal columns results in ipsilateral paralysis or loss of sensation of light touch, proprioception, and vibration. Injury to lateral spinothalamic tract causes contralateral loss of pain and temperature sensation. The anterior spinothalamic tract transmits light touch information so injury to the dorsal columns may result in complete loss of vibration sensation and proprioception but only partial loss of light touch sensation. Vascular Morbidity: Vascular injury may cause a cord lesion at a level several segments higher than the level of spinal injury. As lower cervical spine fracture may result in disruption of the vertebral artery that ascends through the vertebrae so the resulting vascular injury may cause an ischemic high cervical segment of spinal cord injury. At any given level of the spinal cord, the central part is a watershed area.The various groups of SCDs constitute spinal cord syndromes. These syndromes have been classified depending on area affected. These have been elaborated below.Anterior cord syndrome:Anterior cord syndrome (Fig. 4) also known as anterior spinal artery syndrome is caused by insufficient blood in the anterior spinal artery or its total blockage leading to ischemia and infarction of anterior 2/3rd of spinal cord. This ischemia causes dysfunction of corticospinal, spinothalamic tracts and autonomic fibers resulting in loss of motor functions, pain and temperature sensation and hypotension. Anterior spinal artery syndrome is the most common form of spinal cord infarction [10]. 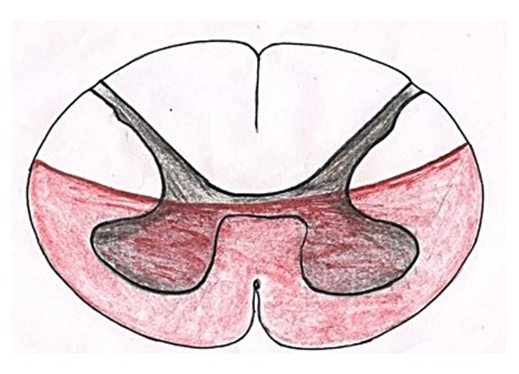 | Figure 4. showing area of spinal cord affected in anterior cord syndrome |
The anterior spinal cord is prone to high risk of infarction because it is supplied by the single anterior spinal artery which has few collateral supplies [11]. As collateral circulation for the anterior spinal artery is scanty, few cord segments (e.g. those around the 2nd to 4th thoracic segments) are vulnerable to ischemia. Injury to an extra vertebral feeder artery or the aorta causes infarction more commonly than do intrinsic disorders of spinal arteries.Central cord syndrome (Fig. 5):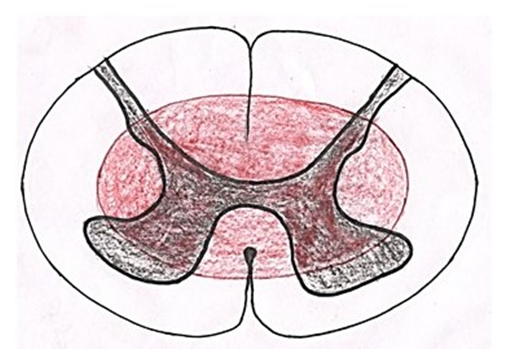 | Figure 5. Showing area of spinal cord affected in central cord syndrome |
It was first described by Schneider et al. in 1954. It is caused by syringomyelia/hydromyelia, trauma, hemorrhage and tumors like ependymoma. In these conditions, the cavity is formed due to destruction of cells and tissues surrounding the central canal causing damage to surrounding tracts. The hemorrhage and tumors associated with central canal compress the tracts causing their dysfunction. In aged individuals, it is associated with hyperextension injury with chronic cervical spondylosis. As fibers of lateral spinothalamic tract cross to opposite side just ventral to the central canal so these are most commonly affected leading to dissociated sensory loss. When spinal lesion extends more laterally, it may cause horner syndrome due to disruption of sympathetic fibers in lateral horn, Kyphoscoliosis due to damage to dorsomedian and ventromedian motor nuclei innervating paraspinal muscles and spastic paralysis due to interruption of corticospinal tract [12]. When the cavity or compression extends dorsally, tract of Goll and tract of Burdach may be damaged causing loss of position and vibratory sense.Brown-Séquard syndrome (Fig. 6):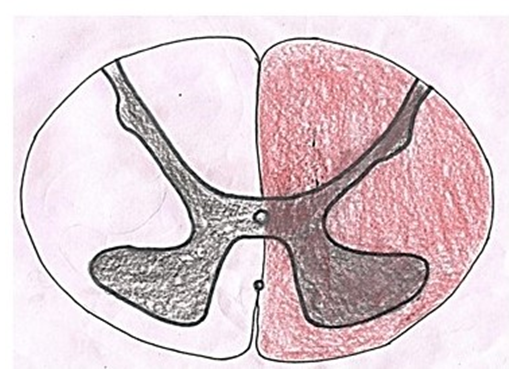 | Figure 6. Showing area of spinal cord affected in brown sequard syndrome |
Charles-Édouard Brown-Séquard described the condition in 1850 for the first time [13]. In this condition, one half of the spinal cord is damaged either by spinal cord tumor/injury or infection resulting in lesion of corticospinal pathway, dorsal column and spinothalamic tracts on the side of the injury. Lesions of these neural structures cause spastic paralysis on the same side of the body below the level of lesion, ipsilateral loss of vibration and proprioception and contralateral loss of pain and temperature.Posterior cord syndrome: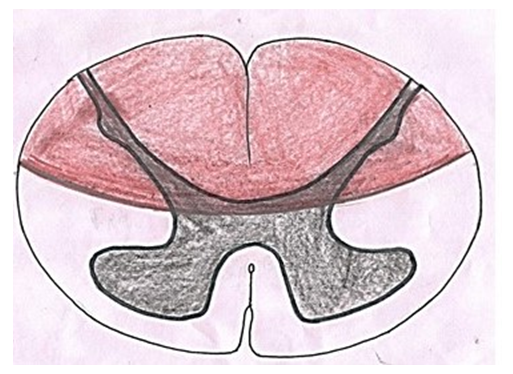 | Figure 7. Showing area of spinal cord affected in posterior cord syndrome |
Posterior cord syndrome a (Fig. 7) also known as posterior spinal artery syndrome is caused due to occlusion of posterior spinal artery culminating into infarction and ultimately dysfunction of posterior column, posterior horn and posterolateral area of lateral column [14]. Besides spinal artery occlusion, it may also be caused by trauma, disc decompression, syphilis etc. [15]. There is degeneration of posterior column and horn and lateral column resulting in interruption of signals from/to the brain. This interruption produces ipsilateral loss of proprioceptive sensation, fine touch, pressure and vibration below the lesion, deep tendon areflexia and in severe circumstances, complete paralysis below the portion of the spinal cord.Cauda equina and conus medullaris syndromes:Affliction of conus medullaris lead to conus medullaris syndrome which may be caused by variety of reasons like lumbar canal stenosis, trauma, tumors to name a few. This syndrome is associated with degeneration of both upper and lower motor neurons resulting in interruption of impulses from/to the brain culminating into bladder and bowel dysfunction, saddle aneasthesia, lower limb weakness, etc. In cauda equina syndrome nerves involved in cauda equina are affected leading to degeneration of lower motor neurons. This syndrome is caused by compression, trauma or tumor in this region. The signs and symptoms are as seen in lower motor neuron lesions and are gradual in onset.In addition to above mentioned syndromes, spinal cord anatomy may be affected in certain conditions like tethered spinal cord, spina bifida and split spinal cord which are elucidated below. Tethered spinal cord:The attachment of spinal cord to inner side of vertebral canal is neurological condition called ‘Tethered spinal cord syndrome’ which is usually associated with spina bifida in children. As the affected children grow, the spinal cord is stretched creating helm of neurological complications. It is secondary to external/iatrogenic injury in adults. In this disease, the spinal cord is stretched during various types of movements. In both cases, neural tissue is damaged interrupting messages to and from the brain creating various neurological signs and symptoms depending on the site and degree of lesion. Due to attachment of spinal cord to the bone, cerebrospinal fluid flow may be blocked leading to syringomyelia worsening the condition further. Split spinal cord: Split spinal cord/diastematomyelia is a congenital defect in which there is longitudinal splitting of spinal cord into two parts either partially or completely by a septum [16]. This anomaly is divided into two groups:TypeI- The spinal cord is divided into two hemicords by bone or fibrous septum. In this type, the longitudinal cleft travels through spinal cord, conus medullaris and also filum terminale [17]. When the split does not reunite distal to the spur, the condition is referred to as diplomyelia, or true duplication of the spinal cord. The hemicords lie within separate dural sacs with two spinal canal and bony or fibrous spurs are usually found between the two sacs [18]. In this type, two pairs of anterior and posterior nerve roots are present. The condition is usually associated with spina bifida and tethering of spinal cord. Each hemicord contains a central canal, one dorsal horn (giving rise to a dorsal nerve root), and one ventral horn (giving rise to a ventral nerve root.) TypeII-. In this type, the spinal cord is incompletely divided into two hemicords which are enveloped in a single dural sac within a single spinal canal [18]. This Condition is known as myeloschisis or cleft spinal cord and is due to failure of the neural plate to form a complete neural tube or rupture of the neural tube after closure. The condition is normally asymptomatic unless accompanied by hydromelia or tethering. In most of the individuals, diastematomyelia of spinal cord is observed between T9-S1 level [19,20]. Cervical diastematomyelia is a very rare entity [21,22]. This condition may be an isolated phenomenon or may be associated with other anomalies of the vertebral bodies such as spina bifida specially meningomyelocele, kyphoscoliosis, hemivertebra and block vertebrae.
4. Conclusions
The knowledge and information enshrined in this work will of be of utmost use to neurosurgeons in carrying out diagnosis and neurosurgical procedures successfully, with least failure rate.
References
| [1] | Saladin Kenneth (2012). Anatomy and Physiology. New York: McGraw Hill. pp. 541, 542. |
| [2] | Purves D, Augustine GJ, Fitzpatrick D, et al., editors. Neuroscience. 2001, 2nd edition. Sunderland (MA): Sinauer Associates; 2001. The Internal Anatomy of the Spinal Cord. Available from: https://www.ncbi.nlm.nih.gov/books/NBK11008/. |
| [3] | Klakeel M, Thompson J, Srinivasan R, McDonald F (2015) Anterior spinal cord syndrome of unknown etiology. Proceedings (Baylor University Medical Center) 28: 85-87. |
| [4] | Prince EA, Ahn SH (2013) Basic Vascular Neuroanatomy of the Brain and Spine: What the General Interventional Radiologist Needs to Know. Seminars in Interventional Radiology 30: 234-239. |
| [5] | Alpagut U, Dayioglu (2002) Anterior spinal artery syndrome after infrarenal abdominal aortic surgery Journal of Cardiovascular Surgery 43: 865-868. |
| [6] | Sliwa JA, Maclean IC (1992) Ischemic myelopathy: a review of spinal vasculature and related clinical syndromes. Arch Phys Med Rehabil 73: 365-372. |
| [7] | Etz CD, Kari FA, Mueller CS, Silovitz D, Brenner RM, et al. (2011) The Collateral Network Concept: A Reassessment of the Anatomy of Spinal Cord Perfusion. The Journal of thoracic and cardiovascular surgery 141: 1020-1028. |
| [8] | Domoto S, Kimura F, Asakura T, Nakazawa K, Koike H, et al. (2016) Intraspinal collateral circulation to the artery of Adamkiewicz detected with intra-arterial injected computed tomographic angiography. Journal of Vascular Surgery 63: 1631-1634. |
| [9] | Heo DH, Cho YJ, Sheen SH, Hong MS, Cho SM, et al. (2010) 3D Reconstructions of Spinal Segmental Arteries Using CT Angiography: Applications in Minimally Invasive Spinal Procedures. American Journal of Neuroradiology 31: 1635-1639. |
| [10] | Schneider, Gregory S. (2010). "Anterior spinal cord syndrome after initiation of treatment with atenolol". The Journal of Emergency Medicine. 38 (5): e49–e52. doi:10.1016/j.jemermed.2007.08.061. |
| [11] | Anterior spinal artery syndrome - Wikipedia. https://en.wikipedia.org/wiki/Anterior_spinal_artery_syndrome. |
| [12] | Quencer RM, Bunge RP, Egnor M, Green BA, Puckett W, Naidich TP, Post MJ, Norenberg M (1992). "Acute traumatic central cord syndrome: MRI-pathological correlations". Neuroradiology. 34 (2): 85–94. doi:10.1007/BF00588148). |
| [13] | Brown-Séquard C É. De la transmission croisée des impressions sensitives par la moelleépinière. Comptesrendus de la Société de biologie, (1850) 1851, 2: 33–44.). |
| [14] | Sebastien Richard, Abdallah Chifaou, Chanson Anne, Foscolo Sylvain, Baillot Pierre-Alexandre, Ducroucp Xavier (2014). "Unilateral posterior cervical spinal cord infarction due to spontaneous vertebral artery dissection". The Journal of Spinal Cord Medicine. J Spinal Cord Med. 37 (2): 233–236). |
| [15] | Mario M, Mirco C, Giampiero F, Fabrizio S, Patrizia N, Nello Q. Posterior spinal artery infarct. AJNR Am J Neuradiol 19: 361-363 1998. |
| [16] | Prasad VS, Sengar RL, Sahu BP, Immaneni D. Diastematomyelia in adults. Modern imaging and operative treatment. Clin Imaging 1995; 19: 270–274. |
| [17] | Singh H, Maurya V, Saini M. Diastematomyelia. Indian J Radiol Imaging. 2000; 10: 255–7. |
| [18] | Castillo M, Mukherji SK. Imaging of paediatric head neck and spine. Philadelphia: Lippincott Raven; 1996. pp. 630–35. |
| [19] | Bruberg JA, Latchaw RC, Emanuel K, et al. Magnetic resonance imaging in occult spinal dysraphism. RSNA. 1988; 26: 181–205. |
| [20] | Byrd SR, Darling CP, McLone DG. Developmental disorders of paediatric spine. RCNA. 1991; 29: 4–5. |
| [21] | Anand AK, Kuchner E, James R. Cervical diastematomyelia: uncommon presentation of a rare congenital disorder. ComputRadiol. 1985; 9: 45–49. |
| [22] | Herman TE, Siegel MJ. Cervical and basicranialdiastematomyelia. AJR Am J Roentgenol. 1990; 154: 806–08. |









 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML