-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Basic Sciences of Medicine
p-ISSN: 2167-7344 e-ISSN: 2167-7352
2020; 9(2): 32-37
doi:10.5923/j.medicine.20200902.03
Received: Oct. 18, 2020; Accepted: Nov. 16, 2020; Published: Nov. 28, 2020

Insecticidal Activity of Ocimum Suave Willd Extracts and Compounds against Sitophilus Zeamais Motschulsky
Sylvia A. Opiyo
Department of Physical and Biological Sciences, Murang’a University, Murang’a, Kenya
Correspondence to: Sylvia A. Opiyo, Department of Physical and Biological Sciences, Murang’a University, Murang’a, Kenya.
| Email: |  |
Copyright © 2020 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Insect pests cause significant loss of maize production in Africa. Among pests of maize, beetles are the most important, with the maize weevil (Sitophilus zeamais Motsch.) and the larger grain borer (Prostephanus truncatus Horn.) being the major pests. The extensive tunneling in maize grain by S. zeamais and P. trancatus adults allows them to convert grain into flour within a very short time. Synthetic pesticides are available in the market. However, such pesticides have adverse effects on non-targeted organisms and also persist in the environment and accumulate in the food chain. Plant have been reported to contain secondary metabolites some of which are toxic to pests as well as pathogenic microorganism The use of botanical in pests and disease control is preferred because they are safe and non-toxic to humans. This study investigated the efficacy of Ocimum suave Willd in management of maize weevil. The essential oil, hexane, ethyl acetate and methanol extracts as well as powdered leaves from the plant exhibited repellence, mortality and growth inhibition activities against the insects. From the isolated compounds, β-sitosterol (1), β-amyrin (3), lupeol (4) and betulinic acid (5) were the most promising in weevil control. Findings from this study revealed that extracts of O. suave are effective against S. zeamais which destroy maize and other cereal grains both in the field and in storage.
Keywords: Maize pest, Sitophilus zeamais, Ocimum suave, Repellence, Mortality, Growth inhibition
Cite this paper: Sylvia A. Opiyo, Insecticidal Activity of Ocimum Suave Willd Extracts and Compounds against Sitophilus Zeamais Motschulsky, Basic Sciences of Medicine , Vol. 9 No. 2, 2020, pp. 32-37. doi: 10.5923/j.medicine.20200902.03.
Article Outline
1. Introduction
- Insect pests cause significant loss of maize in the developing world reducing the 4.9 t/ha world average grain yield production to 1.5 t/ha average in Sub Saharan Africa [1]. An annual average of 20-30% of this little grain is then lost through damage by insect pests in storage [2]. Infestation by post-harvest pests commences in the field but most damage occurs during storage [3]. Among pests of maize in storage, the beetles are the most important, with the maize weevil (Sitophilus zeamais Motsch.) and the larger grain borer (Prostephanus truncatus Horn.) being the major pests in Kenya. The extensive tunneling in maize grain by the insects allows them to convert maize grains into flour within a very short time [3]. Small-scale farmers are often forced to sell maize shortly after harvest to minimize losses during storage, thereby attract low prices and compromising food security at the house hold level.Different pesticides including organochlorines, organophosphates, carbamates, organoarsenicals and organothiocynates have been recommended to control the weevils although they are not accessible by small scale farmers [4]. The role played by these chemicals cannot be ignored. However, some of them had been proved to be environmentally unfriendly because of persistence leading to their accumulation in the environment and gradual absorption into the food chain [5]. Banning some of these chemicals have left a few insecticidal alternatives for pest control operations and even these must conform to a very formidable matrix of desirable factors, which include non-persistence, cost effectiveness, technical effectiveness, low resistance induction in pests, low toxicity to non-target organisms including natural enemy populations, acceptable shelf life and sustainability. Furthermore, insecticides are expensive and mostly out of reach of most smallholder farmers [6]. There is an urgent need to develop safe alternatives that are of readily available, convenient to use and environmentally friendly [7,8].Plants have been reported to contain secondary metabolites some of which inhibit the growth of pests and pathogenic microorganism [9-18]. The use of botanical for pests and disease control is preferred over the conventional synthetic pesticides because they are safe and non-toxic to humans [19-25]. In addition, chances of pests and pathogens developing resistance to botanical pesticides are highly unlikely [7,8]. Plants from the genus Ocimum have antimicrobial, adaptogenic, antidiabetic, hepato-protective, anti-inflammatory, anti-carcinogenic, cardio-protective and insect repellent activities [26-28]. Ocimum suave Willd (Lamiaceae) is a seasonal aromatic shrub which grows specifically at high altitudes, and is used in ethnomedicine to treat ulcers, fever, stomach ache, and bronchopneumonic infections [29]. Essential oil from the plant exhibited insecticidal activity against the brown ear tick - Rhipicephalus appendiculatus [30], the lesser grain borer - Rhyzopertha dominica [31] and housefly - Musca domestica [32]. The present study was conducted to evaluate the efficacy of leaf extracts and compounds from O. suave against of S. zeamais infestation in stored maize grain.
2. Materials and Methods
2.1. Plant Materials
- Ocimum suave leaves were collected from Kitambo region in Kenya. Sample identified was done in Maseno University herbarium by comparison with authentic samples. The plant materials were chopped into small pieces, air dried and ground into fine powder using a mill. Powdered plant material (2 kg) was extracted sequentially with n-hexane, ethyl acetate and methanol by soaking the material in the solvent for seven days with occasional shaking. The mixture was filtered and the solvent evaporated using rotary evaporator to yield 49.5, 70.8 and 120 g of n-hexane, ethyl acetate and methanol extracts, respectively. The extracts were stored at 4°C in brown glass bottles.
2.2. Extraction of Essential Oil
- Fresh leaves of O. suave (2 kg) were cut into pieces and distilled using Clevenger-type apparatus for six hours. The superior phase was collected from the condenser, dried over anhydrous sodium sulfate and kept in a refrigerator (4°C) for further tests.
2.3. Isolation of Compounds
- Hexane extract (40 g) was dissolved in small amount of n-hexane and adsorbed onto silica gel for column chromatography. Fractionation of the extract using gradient of n-hexane-ethyl acetate afforded 200 fractions (20 ml each) whose composition were monitored by TLC using solvent systems n-hexane-ethyl acetate 9:1, 4:1 and 2:1. Fractions with similar TLC profiles were combined resulting into four pools (I-IV). Pool II (fractions 24-76, 18 g) contained two major spots and was further purified using medium pressure chromatography (pressure ≈ 1 bar), eluting with n-hexane-ethyl acetate (9:1 and 4:1) to give β-sitosterol (1, 124 mg) and stigmasterol (2, 88 mg). Pool III (fractions 77-143, 12 g) on subjected to repeated fractionation using n-hexane-ethyl acetate (4:1 and 3:1) yielded stigmasterol (2, 78 mg), β-amyrin (3, 84 mg) and lupeol (4, 65 mg) [13]. Pool IV (fractions 144-200, 7.2 g) gave stigmasterol (2, 24 mg) and lupeol (4, 34 mg). Ethyl acetate extract (40 g) was pre-adsorbed onto silica gel and chromatographed with n-hexane-ethyl acetate gradient to pure ethyl acetate to afford 133 fractions of 20 ml each. The composition of the fractions was monitored by TLC using hexane-ethyl acetate mixtures 4:1, 3:2 and 1:1. Fractions that exhibited similar TLC profiles were combined to constitute two major pools (V and VI). Pool V (fractions 33-79, 17 g) was further purified by chromatography using n-hexane- ethyl acetate (4:1) followed by the same solvent system in the ratio 3:2 to give β-amyrin (3, 53 mg), lupeol (4, 42 mg) and betulinic acid (5, 96 mg). The remaining fractions (pool VI, 6 g) contained one major compound as shown by its TLC profile. The fraction was further purified by chromatography using n-hexane-ethyl acetate (3:2) followed by the same solvent system in the ratio 1:1 to yield betulinic acid (5, 26 mg).
2.4. Mass Rearing of S. zeamais
- Adult weevils (S. zeamais) were obtained from infested maize grains purchased from local market and from this stock, new generation was reared on dry pest susceptible maize grains [33]. Two hundred maize weevils of mixed sexes were introduced into a two liter glass jars containing 400 g weevil susceptible maize grains [34]. The mouths of the jars were then covered with nylon mesh held in place with rubber bands and the jars left undisturbed for 35 days for oviposition. Thereafter, all adults were removed through sieving and each jar was left undisturbed for another 35 days. Emerging adult insects were collected and kept in separate jars according to their age. Adults that emerged on same day were considered of the same age [35].
2.5. Repellency Test
- The test was done according to (Mwangangi and Mutisya [33] with some modifications. Transparent plastic tubings, 13 cm long x 1.3 cm diameter were used as test cylinders. Each test cylinder was plugged at one end with cotton ball containing the test material (leaf powder, essential oil, crude extracts or compounds) from the stem bark of O. suave while the other end was plugged with clean cotton ball which served as control. Actellic dust was used as a positive control. Ten-three-day old unsexed test insects were introduced at the middle of each test cylinder through a hole at the middle portion of the cylinder (0.0 cm) and let to move in any direction of their choice with scoring of distance moved measured in cm using a ruler. The score time was 24 hours after exposure and all tests were done in triplicates.
2.6. Adult Mortality Test
- Contact toxicity assay was done according to Ileke and Oni [36] with some modifications. Toxicity of the test materials (leaf powder, essential oil, crude extracts or compounds) from was tested against adult weevils. The test samples were mixed with talc thoroughly and the dust was admixed with 20 g of maize held in 12 cm high x 6.5 cm diameter glass jars covered with ventilated lids. To ensure a thorough admixture, the grain was put in 12 cm high x 6.5 cm diameter glass jars, dust applied and top lid replaced. The grain was then swirled within the jar until a proper admixture was realized [37]. Twenty-three-day old unsexed insect pairs were then introduced into each dish and exposed to treatments. Actellic dust was used as a positive control and all tests were done in three replicates. Maize weevils were considered dead when probed with sharp objects and there were no responses [36]. The number of dead insects in each vial was counted after 21 days after treatment to estimate maize weevil mortality as follows:
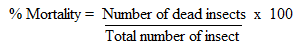 Data on percentage adult weevil mortality were corrected using Abbott’s formula [38]: PT = (Po – Pc) / (100 - Pc); Where PT = Corrected mortality (%); Po = Observed mortality (%); PC = Control mortality (%).
Data on percentage adult weevil mortality were corrected using Abbott’s formula [38]: PT = (Po – Pc) / (100 - Pc); Where PT = Corrected mortality (%); Po = Observed mortality (%); PC = Control mortality (%).2.7. Growth Inhibition Assay
- The test was done according to Ileke and Oni [36] with some modifications. 20 g of clean undamaged and uninfected corn grains were placed in 12 cm high x 6.5 cm diameter glass jars glass jars. Test materials (leaf powder, essential oil, crude extracts or compounds) were thoroughly mixed with the grains in each jar. Crude extracts and pure compounds were mixed with talc thoroughly before being applied to the grains [37]. A mixture of twenty-seven-day old unsexed maize weevils was introduced in each jar and covered with filter paper [35]. The female adults were allowed to oviposit on the seeds for 4 days. On day 5, all insects were removed from each container and the seeds returned to their respective containers. Progeny emergence (F1) was recorded at six weeks (42 days). The containers were sieved out and newly emerged adult weevils were counted [36]. At week six, the grains were reweighed and the percentage loss in weight was determined as follow:
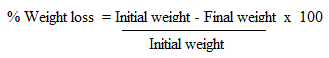
3. Results and Discussion
3.1. Phytochemical Studies
- Chromatographic fractionation of n-hexane and ethyl acetate extracts from O. suave leaves afforded five compounds (Figure 1) namely β-sitosterol (1), stigmsterol (2), β-amyrin (3), lupeol (4) and betulinic acid (5). Structure elucidation of the compounds was earlier reported [21,22,39].
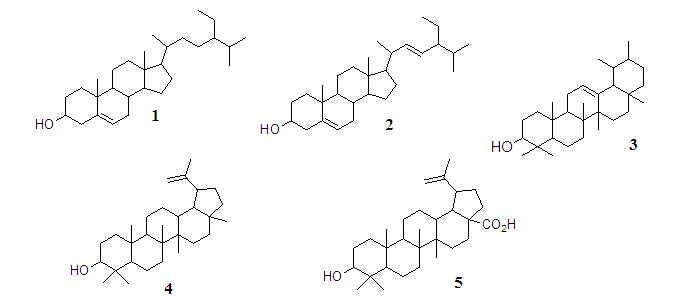 | Figure 1. Compounds isolated from Ocimum suave |
3.2. Repellent Activity
- The repellence activity of leaf powder, essential oil, crude extracts and compounds isolated from O. suave against maize weevil was recorded after 24 hours of exposure duration and the results are presented in Table 1. The repellence activity (distance moved by the weevils away from the center of the tube) varied significantly with the test material used. The oil extract was the most active (mean repellency = 5.9 cm). For n-hexane extract, leaf powder, ethyl acetate extract and methanol extract, the distance moved by the insects away from the center of the tube were 4.3, 3.6, 3.5 and 1.8 cm respectively. This showed that the essential oil, n-hexane and ethyl acetate extracts repelled the insects more than Actellic powder which was used as the positive control. All the isolated compounds caused some repulsion against the weevils. However, all the compounds were less repellent compared to the essential oil, n-hexane and ethyl acetate extracts. Betulinic acid (5) showed significantly higher repellent activity (distance moved = 3.0 cm) compared to the rest of the compounds isolated from O. suave. For β-sitosterol (1) and lupeol (4), the distance moved by the insect were 2.7 an 2.5 cm respectively.
|
3.3. Mortality Activity against Adult Maize Weevils
- The mortality activity of the essential oil, leaf powder, crude extracts and compounds isolated from O. suave against S. zeamais are presented in Table 1. All the tested materials significantly reduce the longevity of adults S. zeamais on treated maize grains and mortality varied significantly amongst the test materials. Essential oil was the most toxic to the weevils and showed 89% mortality at 21 days. Toxicity levels of the crude extracts ranged from 43.5 to 69.4% with n-hexane exhibiting the highest toxicity followed by ethyl acetate extract. All the five compounds isolated from the plant showed varied levels of toxicity to the weevils which were lower compared to those of exhibited by the essential oil, hexane and ethyl acetate extracts. Betulinic acid (5) and β-amyrin (3) gave significantly higher insecticidal activity compared to the other compounds.
3.4. Growth Inhibition Activity and Weight Loss in Maize Grains
- Treatments of maize grains with essential oil, leaf powder, crude extracts and compounds isolated from O. suave significantly reduced the progeny of S. zeamais (Table 1). All the tested materials exhibited growth inhibition activity against the insects. However, the growth inhibition activity exhibited the plant materials were lower compared to Actellic powder which gave 100% growth inhibition. The adult insect emergence was lowest in grains treated with the essential oil (adult emergence = 7.25%) followed by the leaf powder, n-hexane extract, ethyl acetate extract and methanol extract having adult emergence of 8.4, 10.4, 12.35 and 17.9% respectively. Insect emergence from grains treated with the compound isolated from O. suave ranged between 23.7 to 53.2% with β-sitosterol (1) and β-amyrin (3) treated grains having 23.7 and 26.1% insect mergence respectively. Treatment of maize grains with the plant extractives and compounds significantly reduced the weight loss due to the insect infestation. The best protection of the grains from weight loss was obtained from the oil extract and leaf powder giving 5.8 and 8.3% weight loss respectively. Hexane, ethyl acetate and methanol extracts recorded 8.3, 6.7 and 17.5% weight loss, respectively.Repellent, mortality and adult emergence inhibition test have shown that the oil from O. suave is the most effective in controlling maize weevil (S. zeamais). The findings were in agreement with previous studies which reported the efficacy of essential oils from various plants in controlling insect infestation [32,40,41]. The leaf powder was also effective in controlling the weevil and this is in agreement with previous reports [42,43] indicating the use of various plant leaves to manage storage grain insect pests. From the isolated compounds, β-sitosterol (1), β-amyrin (3), lupeol (4) and betulinic acid (5) were the most promising in weevil control. From the results, the isolated compounds are less active compared to the essential oil and crude extracts in the repellence, adult mortality as well as in the growth inhibition tests suggesting possible synergistic effect in the extracts. This suggests that insect pests can be managed using herbal extracts as had also been observed in other studies [23,24,44,45]. Use of plant extracts as bio-pesticides is environmentally safe compared to the chemicals.
4. Conclusions
- Findings from this study revealed that extracts of O. suave have repellent, toxicity and growth inhibition activities against S. zeamais which destroy maize and other cereal grains both in the field and in storage. Among the isolated compounds, β-sitosterol (1), β-amyrin (3), lupeol (4) and betulinic acid (5) were the most promising for the weevil control. Further research aimed at isolation of insecticidal principles from the more polar (methanol) extracts is necessary.
ACKNOWLEDGEMENTS
- The authors are grateful to the National Commission for Science, Technology and Innovation (NACOSTI) and Biosciences Eastern and Central Africa Network (BecANet) for financial support. We also acknowledge the assistance from Chemistry Department, Maseno University.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML