-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Basic Sciences of Medicine
p-ISSN: 2167-7344 e-ISSN: 2167-7352
2020; 9(1): 1-7
doi:10.5923/j.medicine.20200901.01

Repellent Properties of Compounds and Blends from Nigella sativa Seeds Against Anopheles gambiae
Ephantus G. Ndirangu 1, 2, Sylvia A. Opiyo 1, Margaret W. Ng’ang’a 2
1Department of Physical and Biological Sciences, Murang’a University, Murang’a, Kenya
2Department of Chemistry, Kenyatta University, Nairobi, Kenya
Correspondence to: Sylvia A. Opiyo , Department of Physical and Biological Sciences, Murang’a University, Murang’a, Kenya.
| Email: |  |
Copyright © 2020 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Malaria, transmitted to humans by the bites of infected Anopheles mosquitoes is a public health concern. Some available repellents against the insect have adverse effects on humans and environment. This study determined Anopheles gambiae repellent compounds and blends from Nigella sativa L. seed. From GC-EAD analysis, repellent compounds were 1,2,3,4,5-pentamethylcyclopentane, α-thujene, α-pinene, β-pinene, tetradecane, p-cymene, α-longipinene and longifolene. Repellency of blend 1 consisting of six GC-EAD active compounds was lower than that of essential oil and DEET. Subtraction of p-cymene and (-)-α-pinene from blends 2 and 6 respectively significantly increased repellency while subtraction of (+)-β-pinene, (-)-β-pinene, (+)-α-pinene, tetradecane or 1,2,3,4,5-pentamethylcyclopentane significant decreased repellency of the blends. Blends 2 and 6 repelled 100% of mosquitos at 0.1g/ml while (+)-α-pinene repelled 99.38%. These findings confirm that essential oil of N. sativa L. seeds contain compounds that repel An. gambiae. The positive enantiomer of α-pinene was the most repellent which confirms the compound to be the main active ingredient the oil.
Keywords: Nigella sativa, Essential oil, Anopheles gambiae, Repellence, GC-EAD
Cite this paper: Ephantus G. Ndirangu , Sylvia A. Opiyo , Margaret W. Ng’ang’a , Repellent Properties of Compounds and Blends from Nigella sativa Seeds Against Anopheles gambiae, Basic Sciences of Medicine , Vol. 9 No. 1, 2020, pp. 1-7. doi: 10.5923/j.medicine.20200901.01.
Article Outline
1. Introduction
- Malaria, a vector-borne disease transmitted to humans by the bites of infected Anopheles mosquitoes, is a public health concern especially in Africa [1]. Malaria occurs mostly in poor tropical and subtropical areas of the world. In many of the countries affected by malaria, it is a leading cause of illness and death. In 2016, malaria caused an estimated 216 million clinical episodes and 445,000 deaths worldwide with about 90% of deaths occurring in African [2]. One of the best ways to deal with this global enemy is to prevent mosquito bites using physical and chemical barriers, treatment of fabric with toxicants, and the use of topical (skin) repellents [3]. N,N-dimethyl-3-methylbenzamide (DEET) has been the mainstay among repellents approved for use on human skin [4] because it has broad-spectrum activity and effectively repels mosquitoes as well as other insects including flies, chiggers, fleas, and ticks [4]. Due to side effects and the adverse effects on the environment and food chain [5-7], the use some of the synthetic chemicals has been banned. Previous studies have shown that in plants there are bioactive compounds that have gained increasing interest as potential therapeutic agents [8-11]. Extracts from numerous plants species have been shown to possess repellent and toxic effects against insects [12-18]. Several plants have been studied as possible mosquito repellents revealing the existence of natural repellents with good efficacy [19-21]. Insect repellents from plant origin are preferred since they are more friendly to both the user and the environment.Nigella sativa L. (also called black cumin, or black seed) is an annual herb of the Ranunculaceae family and is cultivated in various parts of the globe [22]. N. sativa L. seeds have been used traditionally to treat of asthma, fever, cough, eczema, headache, rheumatism, influenza and bronchitis [23]. Previous studies have shown that extracts and oils from N. sativa L. seed to have anti-inflammatory, antimicrobial and antioxidant activities [24,25]. Extracts from the plant also exhibited insecticidal and insect repelling activity against Amblyomma americanum, Tribolium castaneum, Tuta absoluta and Mosquito [26,27]. Most researchers have concentrated more of determining the bioactivity of the crude extracts and oils from the plant on the plant. The main components of N. sativa seed essential oil are p-cymene, thymol, thymoquinone and 9-eicosyne [24,28]. The objective of the present study was to determine the mosquito repellence efficacy of compounds and blends from Nigella sativa seed essential oil.
2. Materials and Methods
2.1. Plant Material
- Dry N. sativa seeds were obtained from the Kenyan local market. The seeds were authenticated at the Department of Botany, Kenyatta University in Kenya. The sample was cleaned and freed from dust and foreign material. A weight of 125 g of N. sativa seeds were ground using an electric house-hold spice grinder.
2.2. Extraction of Essential Oil
- The ground N. sativa seeds (125 g) were transferred into a clean flask and 500 ml of distilled water was added. 10 g of sodium chloride was added to the mixture to reduce the foaming during boiling. Oil extraction was done by hydro-distillation method using the Clevenger-type apparatus [29]. The apparatus were set-up and the mixture were heated at 100°C for three hours. The oil was separated from the aqueous phase and diluted with pure acetone to make a concentration of 10%, which was stored in amber-colored vial at -4°C for phytochemical analysis and bio-assays.
2.3. Mosquito Rearing
- Anopheles gambiae s.s. were obtained from colonies reared in the insectaries at International Centre of Insect Physiology and Ecology (ICIPE, Nairobi, Kenya) and were reared according to the WHO protocol [30]. Mosquito eggs were hatched by simultaneously flooding the moist filter paper platforms. Rearing was carried out in the insectary maintained at 27-28°C and approximately 80% humidity on a 12h/12h light and darkness cycle and maintained at optimal larval concentrations to avoid possible effects of competition. Mosquito larvae were fed on ground baby fish food while adults were offered a fresh 10% (w/v) sucrose solution meal daily and on hamsters as a source of blood meals when required to produce eggs.
2.4. Chemicals
- Standards were purchased from Sigma-Aldrich namely (+)-β-pinene, (-)-β-pinene, (+)-α-pinene, (-)-α-pinene, p-cymene, α-longipinene, tetradecane and 1,2,3,4,5-pentamethylcyclopentane. The purchased chemicals were of analytical grade purity.
2.5. Gas Chromatography with Electroantennographic Detector (GC-EAD)
- GC-EAD analysis was used to isolate EAD-active components from N. sativa seeds essential oil. GC-EAD tests were performed on HP 5890 series II gas chromatograph equipped with a flame ionization detector (FID) and HP Ultra 1 (cross-linked methyl silicon gum) capillary column (50 m × 0.2 mm × 0.33 µm) using nitrogen at a flow rate of 0.8 ml/min as the carrier gas. The oven temperature was programmed from 60°C for 5 min and then 5°C /min to 280°C, where it was held for 15 min. Antennae of thirty (30) laboratory reared 5-7 days old females of An. gambiae were used. A glass micro-pipette containing Beadle-Ephrussi saline was inserted through the head of the insect. The fine tip of the micro-pipette was pushed through the head between the thorax and the head of the insect. The other end of the micro-pipette was sheathed over a silver wire, the recording electrode, which was connected to the input of a universal AC/DC UN-05 amplifier (Syntech, The Netherlands). To complete the circuit, the distal end of the antenna was nipped off with a scalpel and the open end inserted into similar glass micro-pipette containing the saline and was sheathed over a silver wire electrode that was grounded. The effluent from the capillary column was split in a ratio of 1:1 into two 50 cm long de-activated silica columns, one connected to the FID and the other connected to a stainless-steel tube (5mm i.d.) that was focused onto the antennal preparation. A make-up gas (40 ml/min) was added just before the split point to accelerate the effluent through deactivated columns. The deactivated transfer line carrying the effluent over the antennal preparation was maintained at 150°C by a THC-3 temperature control unit (Syntech, The Netherlands). Aliquots (8-10 µl) of N. sativa seeds essential at concentration of 0.1% by mass were analyzed by the GC-EAD and GC signals were monitored synchronously using a program on a GC/EAD interface card (Syntech, The Netherlands) installed in a PC (Harvard Professional computer, American Megatrends Inc.).
2.6. Gas Chromatography - Mass Spectrometry (GC-MS)
- The antennal active peaks from the essential oil of N. sativa seeds were identified by GC-MS on a GC-MS HP 8060 series II GC coupled with a VG Platform II mass Spectrometer. The MS was operated in the EI mode at 70 eV and an emission of 200 µA, with the temperature of the source held at 180°C multiplier voltage at 300 V. The MS had a scan cycle of 1.5 s (scan cycle of 1 s and inter-delay of 0.5 s) and scan ranges m/z 38-650. Helium was used as the carrier gas and the column temperature, 50°C for 5 min, rising to 90°C at 5°C min-1, then 200°C at 2°C min-1, and then rising 280°C at 20°C min-1 held for 20 min. The compounds were identified by analysis of their mass spectra and direct comparison with the Institute of Standards Technology libraries 98.1 (NIST) and Wiley Registry of Mass Spectral Data, 8th edition database of library of mass spectra, on the GC-MS equipment.
2.7. Repellency Bioassay
- Tests were done according to WHO protocol [30] on An. Gambiae s.s. Repellency assays were done with 5-7 days old females of An. gambiae that had been starved for 18 hours but previously fed on 6% glucose solution [31]. Subtractive bioassays were done to identify constituents that contributed significantly to the repellence of the blend of EAG-active compounds. The essential oil from N. sativa seeds, GC-EAG-active compounds and blends of the GC-EAG-active compounds were tested at concentrations of 10-5, 10-4, 10-3, 10-2 and 10-1. The tests were performed on 6 human adults (18yrs ≤ age ≤ 50yrs). Participants had no contact with lotions, perfumes oil or perfumed soaps on the day of the experiment. An insect bite cream was provided to the participants in case of any minor bites and associated irritations. A total of 18 cages each measuring 50×50×50 centimeters were used [31]. Test solutions (1.0 ml) were dispensed on one of the forearms of a volunteer from wrist to the elbow covering an area of 500 cm2. The rest of the hand was covered with a glove. Acetone was dispensed on the other forearm to serve as control. The control and treated arms were interchanged regularly to eliminate bias [31]. The control arm was first introduced into the cage immediately after releasing the 50 experimental mosquitoes and kept there for 3 minutes. The number of insects that landed on control arm during the test was recorded. The treated arm was then introduced into the cage for the same period of time and the number of landing insects recorded. The different concentrations of the sample and DEET were tested starting with lowest concentrations. For each test, six pairs of arms were used. Percentage protective efficacy (PE) was calculated using the formula PE = (C-T/C) ×100% where C and T are the mean numbers of mosquitoes that landed on the control and the test arm respectively [32]. Dose-response relationship was determined using probit analysis and repellent doses at RD50 and RD75 values obtained from regression model [32].
2.8. Ethical Issues
- Ethical approval for this study was given by Kenyatta University Ethics Review Committee, Kenya with the reference number of KU/R/COMM/51/16. The approval was given after submitting the detailed proposal of the study to the Committee for thorough review. The volunteers were given written consent forms which they were taken through before signing in presence of a witness who was not a participant in study participant.
2.9. Data Analysis
- The data obtained was subjected to analysis of variance (ANOVA) and means ranked using Student-Newman-Keuls (SNK) at the 5% significance level.
3. Results and Discussion
3.1. Gas Chromatography/Electro-Antennographic Detection Using An. gambiae
- Hydro-distillation of the seeds of N. sativa gave a dark yellow colored oil (0.68 g) with a characteristic odor. The GC-EAD analysis of the revealed that eight compounds consistently elicited electrophysiological responses from the antennae of the female An. gambiae (Figure 1). The compounds were identified by GC-MS by comparing their fragmentation patterns using Institute of Standards Technology libraries 98.1 (NIST) and Wiley Registry of Mass Spectral Data, 8th edition (Figure 2). The compounds were identified as 1,2,3,4,5-pentamethylcyclopentane (1, Rt 9.13 min), α-thujene (2, Rt 9.38 min), α-pinene (3, Rt 9.49 min), β-pinene (4, Rt 10.41 min), tetradecane (5, Rt 10.88 min), p-cymene (6, Rt 11.42 min), α-longipinene (7, Rt 16.57 min) and longifolene (8, Rt 17.33 min) and the structures are given in Figure 3. The results are in agreement with previous studies which reported the insecticidal and insect repellent activities of the compounds against various insects [33]. Both isomers of compound 3 [(-)-α-pinene and -(+)-α-pinene] repelled house fly, Musca domestica, [33] while in another studies the compounds also showed larvicidal activity against Anapheles subpictus, Aedes albopictus, Culex tritaeniorhynchus [34]. p-Cymene (6) showed repellent activity against Amblyomma americanum L. and Aedes aegypti L. [35]. Longifolene (8) showed termiticidal and antifeedant activities against Reticulitermes speratus Kolbe [36].
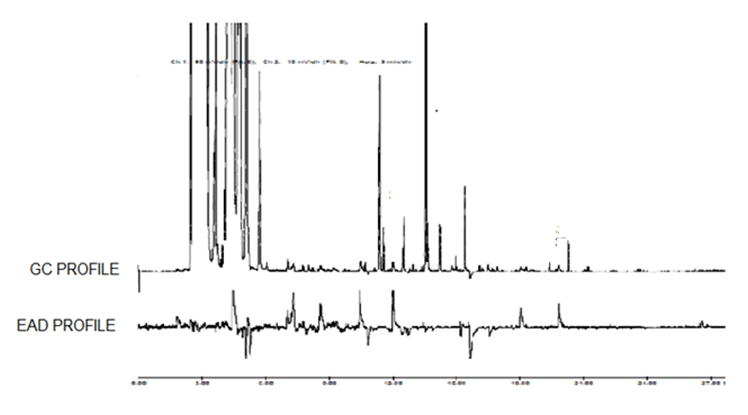 | Figure 1. Gas chromatography-electroantennographic detection (GC-EAD) using the antennae of Anopheles gambiae females in response to Nigella sativa L. seed essential oil |
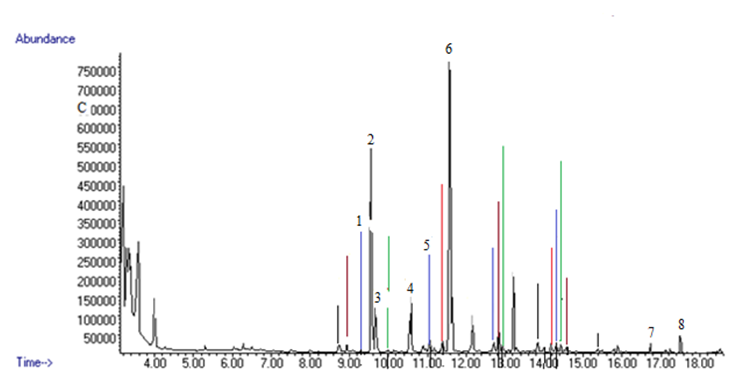 | Figure 2. GC-MS Chromatogram of essential oil from N. sativa seeds |
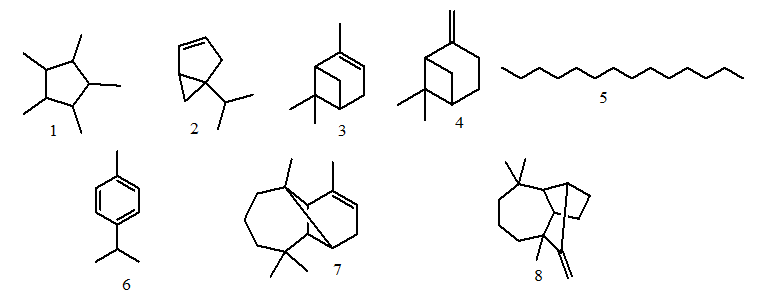 | Figure 3. GC-EAD active compounds from Nigella sativa essential oil |
3.2. Repellency of Compounds and Blends from Nigella sativa
- The repellence activity of essential oil, selected standards [(+)-β-pinene, (-)-β-pinene, (+)-α-pinene, (-)-α-pinene, p-cymene, α-longipinene, tetradecane and 1,2,3,4,5-pentamethylcyclopentane] and blends of the standards were determined. Mean percent repellency at different doses and RD50 and RD75 values are given in Table 1. All the tested drugs exhibited concentration dependent activities against An. gambiae. At higher doses the percent repellence of the essential oil was comparable to that of DEET. The essential showed 98.81% repellence at 0.01g/ml while at 0.1g/ml it gave 100% repellence against the mosquito.
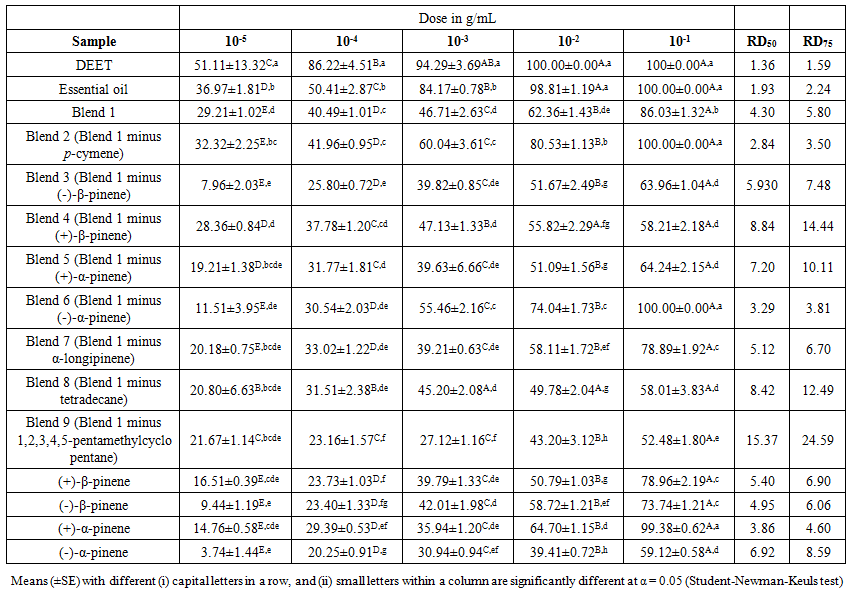 | Table 1. Mean percentage repellency (+SE) of blends and standards at different concentrations |
4. Conclusions
- Essential oil of N. sativa seeds contains compounds that repel An. gambiae. The repellency of the oil and those of blends 2 and 6 were comparable to the positive standard. The results from the present study lays down groundwork for downstream development of appropriate blends for personal and space protection against An. gambiae.
ACKNOWLEDGEMENTS
- The authors acknowledge ICIPE for allowing us to perform GC-MS, GC-EAD, mosquitoes repellency assays and providing us mosquitoes for bio-assay in their institution. We also acknowledge Mr. Xavier Cheseto (ICIPE) for running GC-FID and GC-MS, Mr. Vincent Odhiambo (ICIPE) who assisted in GC-EAD and Mr. Richard Ochieng (ICIPE) who assisted in carrying out repellence assays. We also thank the volunteers for their assistance in repellent tests.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML