-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Basic Sciences of Medicine
p-ISSN: 2167-7344 e-ISSN: 2167-7352
2019; 8(1): 1-11
doi:10.5923/j.medicine.20190801.01

Types of Wild Plant of Asteraceae Family Potentially as Herbicide in Brawijaya University
Dewi Krisdiyanti1, Jati Batoro2
1Bachelor Degree, Department of Biology, Brawijaya University, East Java, Indonesia
2Department of Biology, Faculty of Mathematics and Natural Sciences, Brawijaya University, East Java, Indonesia
Correspondence to: Dewi Krisdiyanti, Bachelor Degree, Department of Biology, Brawijaya University, East Java, Indonesia.
| Email: |  |
Copyright © 2019 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Asteraceae is one of the plant family that has the number of species reaches 30.000 species and spread all over the world including in the University of Brawijaya. The University is located on Veteran Street, Malang Town and has an area of 2.203.948 m². This research is important to know the species of Asteraceae family that have potential as herbicide in Brawijaya University Campus. The method used is roaming by conducting surveys directly in the field. The method used is roaming by conducting surveys directly in the field. The samples were then identified in the Laboratory of Plant Taxonomy. Further identification is made in the form of parallel keys. It was then determined that herbicide species were potential herbicides based on literature studies. Data analysis was done by using descriptive method. The results of this study found there are 13 species namely Argeratum conyzoides, Galinsoga parviflora, Crassocephalum crepidioides, Calyptocarpus vialis, Sonchus arvensis, Eclipta prostrata, Synedrella nodiflora, Tridax procumbens, Emilia sonchifolia. Nine species have the potential to be herbicides. Four species including Youngia japonica, Acmella paniculata, Vernonia cinerea and Senecio vulgaris, do not yet have information about their potential as herbicides.
Keywords: Asteraceae, Brawijaya University, Survey, Herbicides
Cite this paper: Dewi Krisdiyanti, Jati Batoro, Types of Wild Plant of Asteraceae Family Potentially as Herbicide in Brawijaya University, Basic Sciences of Medicine , Vol. 8 No. 1, 2019, pp. 1-11. doi: 10.5923/j.medicine.20190801.01.
Article Outline
1. Introduction
- Asteraceae is one of the largest plant species in the world. Asteraceae is known as sunflower because this family member has flowers that are shaped like sunflowers. The benefits of Asteraceae are commonly used as traditional medicines for certain diseases or to heal wounds. Asteraceae is also consumed as fresh vegetables, animal feed, and ornamental plants, and can be used as hair fertilizers [34]. Besides being used as a traditional medicine, and also be used as a natural herbicide because some species have allelochemical content. [6] Fourteen families’ poisonous plants for example Euphorbiaceae (eight species), Asteraceae (six species), Solanaceae (six species) etc.Allelochemicals have toxic properties that can inhibit nutrient uptake and the growth of shoots and other plant roots [1]. The Asteraceae are invasive, usually containing allelochemistry so that they can inhibit the growth of other plants around them. The Asteraceae belong to plants that are very easy to grow, because pollination can occur easily through wind assistance. If the seeds are scattered and fall in the appropriate area, the seeds will grow and develop. Therefore, the plants of the Asteraceae tribe are very easy to find, one of which is on the campus of Brawijaya University (UB).Brawijaya University (UB) is one of the universities in Malang City and was established on January 5, 1963 [36]. Brawijaya University (UB) is located at Jl. Veteran Malang town and has an area of 2.203.948 m² [35]. Wild plants are found on the UB campus, especially from the Asteraceae tribe. Locations commonly found in wild plants are in the parking area, on the side of the main road and around the area of faculties. Many types of plants from the Asteraceae are found, some species may have potential as natural herbicides. Therefore, this research is important to inventory the species of the Asteraceae family on the Universitas Brawijaya campus. Morphological description and form the key to the determination of each species and determine the plant species of the Asteraceae which has the potential as herbicides.
2. Materials and Methods
2.1. Time and Place
- This research was conducted in December 2017-July 2018. Sampling was carried out at the University of Brawijaya campus. Data analysis was conducted at the Taxonomy Laboratory, Department of Biology, Faculty of Mathematics and Natural Sciences, Brawijaya University, Malang, East Java Indonesia.
2.2. Plant Sampling
- Plant sampling is carried out with a direct survey on the campus of the University of Brawijaya which aims to inventory all types of Asteraceae that exist. When found in the Asteraceae plants, documentation is first done by taking photos using the camera, then marking the location with GPS. Plants are taken and collected, the part of the plant taken must include the roots, stems, leaves, flowers, fruits and seeds. The plants that have been taken are taken to the Laboratory of Plant Taxonomy for identification.
2.3. Plant Identification
- Identification is done by observing the morphological structure of the plant species. Morphology observed in the form of roots, stems, leaves, flowers, seeds and fruit. Roots are observed in root systems. The stem is observed in the stem surface using a loup, the length of the stem and branch is measured by a meter or ruler, and the shape and type of the stem is observed. Leaves were observed in the abaxial and adaxial parts using loup. The length and width of the leaf is measured using a meter or ruler. Leaf edges, leaf bones, leaf shape, leaf base, apical leaves, leaf attachment, leaf arrangement, and inflorescence were also observed. The organ of the flower was observed using a stereo microscope to see the size of the flower. Flower organ surgery is done by tweezers to determine the number of stamen, stamen arrangement, fusion stamen, number of pistils, number of styles, number of stigma, style, ovary position and fruit of the Asteraceae. Documentation was carried out using a camera, the description obtained was matched with the book Flora Of Java [4] to determine the name of a plant species and the book Plant Identification Terminology An Illustrated Glossary [19] to describe plant morphology, while also using various other plant identification books and official websites such as Plantlist.
2.4. Making Herbarium
- The manufacture of herbarium is done after identification of each type of plant. Plants that have been collected must be in accordance with the paper size of the installation of a standard herbarium, 11½ x 16 ½ inches. If the plant is small, all parts of the plant are collected, while for large plants must include parts of plants that show growing habits [39]. The sample is wrapped with newspaper and then presses using sasak for oven or drying. The plants that have been finished pressed with sasak are then put in the oven at 55 °C for 2-3 days, after drying the sample is taken from the oven and starts mounting on the herbarium paper. Mounting is done using tape to attach plants to the herbarium paper. The plants that have been finished mounting are then labeled with a description of the plant, the location of the harvest, the date of collection, the collection number, the name of the collector, and the name of the plant species. Herbarium is stored in the Herbarium Brawijaya University (MUBR) [7].
2.5. Literature Study
- The literature study aims to find information about the potential of the Asteraceae family as a natural herbicide. Literature study is done by searching and reading various journals and scientific articles that discuss natural herbicides derived from the Asteraceae.
2.6. Creating Parallel Keys
- The results of the identification that has been done, then made into a pair of statements (couplet). Each statement is made against each other. At the end of each statement given a number that will show the next direction, this step is carried out repeatedly until the final statement shows the name of the type [6].
2.7. Data Analysis
- Data analysis is done by descriptive method. The results of the identification of each specimen that has been carried out are presented in the form of morphological descriptions. Morphological descriptions include the type of root, stem, leaf, flower, and fruit.
3. Results and Discussion
3.1. Character Description of Asteraceae on the University Campus
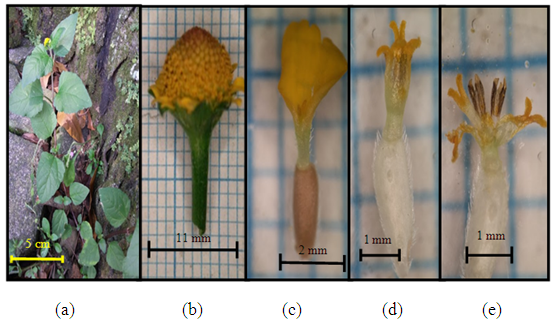
- BrawijayaBased on the results of the field survey, 13 namely Asteraceae families were found, consisting of Ageratum conyzoides, Youngia japonica, Galinsoga parviflora, Calyptocarpus vialis, Sonchus arvensis, Crassocephalum crepidioides, Eclipta prostrata, Acmella paniculata, Synedrella nodiflora, Tridax procumbens, Emilia sonchifolia, Vernonia cinerea, and Senecio vulgaris.Ageratum conyzoides L. Sp. Pl. 839 (1753) Flora of Java. 10: 376 (1968)Synonym: Ageratum hirsutum Lam., in Poir., Encycl. Suppl. 1: 242 (1810).Ageratum latifolium var. latifoliumAgeratum odoratum Vilm., Fl. Pl. Terre, ed. 2 : 42 (1866)Terrestrial. Annual herbaceous with taproots. The stem is green-brown. Shape of the nodose rod. Stem surface has trichomes; length about 15-21 cm, diameter 0.13 to 0.21 cm. Shape of the leaf ovate, the base rounded. Leaf tip acute, including single leaves. The leaf bone reticulate, the edge of the leaf shaped like crenate. The attachment of petiolate, leaves arranged opposite or facing each other. Surface of flower purple, inflorescence capitulum (Figures 1a and 1b). The crown is tubular and there are five indentations (Figure 1c). The number of stamen is five and arranged in included. Stamen fusion coalescent, the stamen has a white color. Pistil and style number one and the forked stigma (Figure 1d); style macrostylous (style longer than pistil). Fruit achene. Ovary inferior.Distribution and habitat: Ageratum conyzoides originates from Central America and the Caribbean, these plants have spread in several parts of the world [22]. This plant distributed in West Africa, Australia, Colombia, Costa Rica, Ecuador, Fiji, French Polynesia, Guam Islands, United States (Hawaii Islands), Tonga, Vanuatu, Palau, Mauritius, Nicaragua, Solomon Islands, Papua New Guinea, Samoa and Southeast Asia (including China, India, Philippines, Singapore, Thailand, Vietnam, Cambodia, Malaysia and Indonesia (Malang), Brazil and Korea. The habitat of Argeratum conyzoides is in grasslands, forests, waterways, and vacant land [21].Specimen examined: dk’s collection of 01 (MUBR) forest; 09 (MUBR).Local name: Wedusan (East Java, Malang) and Babandotan (West Java).
 | Figure 1. Ageratum conyzoides L. (a) Plant individuals; (b) the size of one flower hump; (c) tube flowers (d) pistil and stamen |
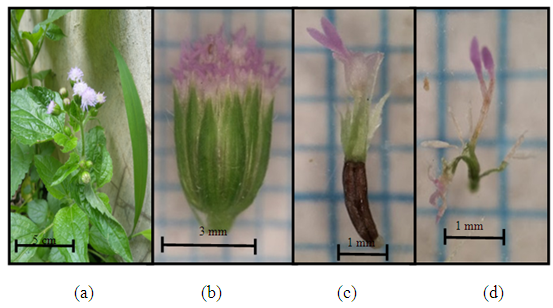
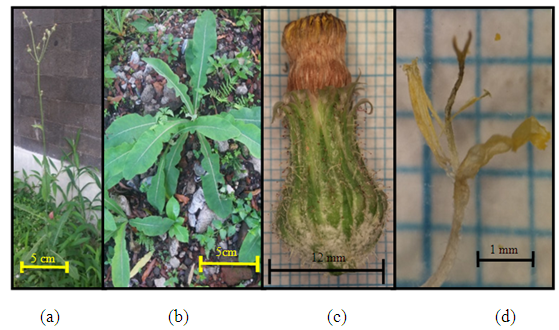
 | Figure 2. Youngia japonica (L.) DC (a) Plant individuals; (b) the size of one flower hump; (c) pansy flower (d) pistil and stamen |
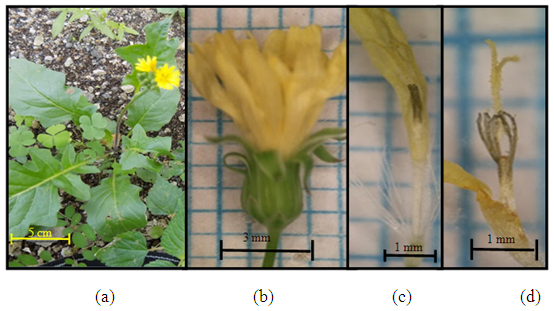
 | Figure 3. Galinsoga parviflora Cav (a) Plant individuals; (b) the size of one flower hump; (c) female flowers (flowers edge (d) tube flower (effeminate flower) (e) pistil and stamen |
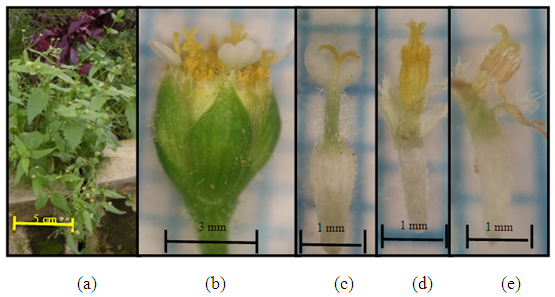
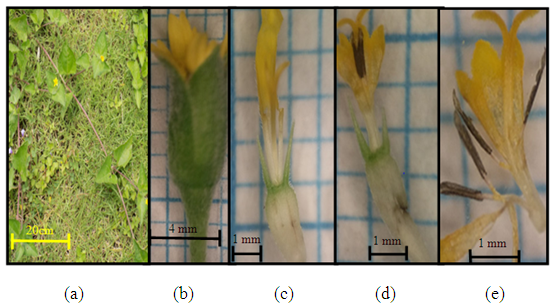 | Figure 4. Calyptocarpus vialis Less. (a) Plant individuals; (b) the size of one flower hump; (c) female flower (edge flower (d) tube flower (effeminate flower) (e) pistil and stamen |
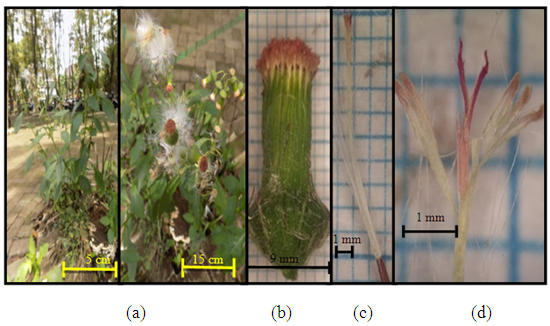
 | Figure 5. Sonchus arvensis L. (a) Plant individuals; (b) plants when in the form of rosettes; (c) size of one flower (d) pansy flower (e) pistil and stamen |
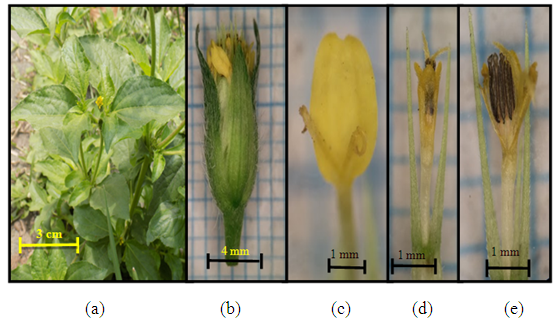
 | Figure 6. Crassocephalum crepidioides (Benth.) S. Moore (a) Plant individuals; (b) the size of one lumpy flower; (c) tube flowers (d) pistil and stamen |
 | Figure 7. Eclipta prostrata (L.) L. (a) Plant individuals; (b) the size of one lumpy flower; (c) edge flowers (female flowers (d) pistil and stamen (tube flowers) |
 | Figure 9. Synedrella nodiflora (L.) Gaertn. (a) Plant individuals; (b) the size of one lumpy flower; (c) edge flowers (female flowers (d) tube flowers (e) pistil and stamen (tube flowers) |
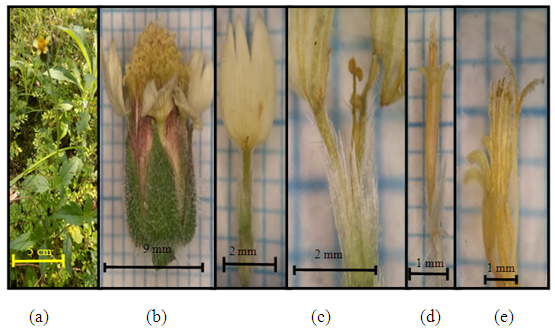 | Figure 10. Tridax procumbens L. (a) Plant individuals; (b) the size of one lumpy flower; (c) edge flowers (female flowers (d) tube flowers (e) pistil and stamen (tube flowers) |
 | Figure 11. Emilia sonchifolia (L.) DC. (a) Plant individuals; (b) the size of one lumpy flower; (c) tube flowers and (d) pistil and stamen from tube flowers |
 | Figure 12. Vernonia cinerea Less (a) Plant individual; (b) the size of one lumpy flower; (c) tube flowers (d) pistil and stamen from tube flowers |
 | Figure 13. Senecio vulgaris L. (a) Plant individuals; (b) the size of one lumpy flower; (c) tube flowers (d) pistil and stamen from tube flowers |
3.2. Key to Determination
- The key to determination is based on the characteristics of each plant that has been found on the campus of Brawijaya University. The following are identified characters:1a. Leaf deltoid. Flower ligula absent, inflorescence capitulum ................................ Ageratum conyzoides1b. Leaf deltoid. Flowers with ligule, inflorescence capitulum ............................................................ 22a. Thick stem, smooth stem surface ........................................................................................... 32b. Small stem, rough stem surface ....................................................................... Calytocarpus vialis3a. Leaf thick dark green, the surface slippery ...................................................... Acmella paniculata3b. Leaf light green, arrangement rosette ....................................................................................... 44a. Leaf attachment petiolate, the apical obtuse ........................................................ Youngia japonica4b. Leaf attachment decurrent, virgate, the apical lyrate ................................................................. 55a. Flower yellow, leaf length 9 - 33 cm ...................................................................... Sonchus arvesis5b. Flower purple, leaves are smooth ............................................................................................ 66a. Leaf in base attenuate, leaf pinni-palmate ................................................................................ 76b. Leaf auriculate, reticulate, leaf decurrent ............................................................. Emilia sonchifolia7a. The leaf arrangement dextrorse, the abaxial and adaxial part of the leaf smoot .......... Vernonia cinerea7b. The leaf arrangement opposite, the abaxial and adaxial part of the leaves have trichomes .............. 88a. The number of stamen 4, the style bifid .............................................................. Eclipta prostrata8b. The number of stamen 5, the style macrostylous ...................................................................... 99a. Apical leaves acute, leaf lanceolate ............................................................... Galinsoga parviflora9b. Apical leaves aristulate, leaf attenuate ................................................................................... 1010a Flower tube yellow. The stem trichomes ................................................................................. 1110b Fower tube brownish-red. Stem surface smooth .................................. Crassocephalum crepidioides11a. Leaf bone cauline. Flower capitates .................................................................... Senecio vulgaris11b. Leaf bone pinni-palmate, leaf arrangement opposite ................................................................. 1212a Stem tetragonal, leaf ovate, inflorescence verticillaster ................................. Synedrella nodiflora12b. Stem procumbens, leaf elliptic, inflorescence capitulum .................................. Tridax procumbensThe key to this determination is a practical key that can be used in the field, especially at the UB campus. The guidelines used in making the key to this determination are based on the guidelines contained in the Flora of Java [4].
3.3. Potential of Herbicides of Some Asteraceae Tribes at Universitas Brawijaya (UB) Campus
- The Asteraceae have a lot of potential, one of which has a herbicide. There are nine types of Asteraceae plants found on environment UB campus that have potential as herbicides. These plants include Argeratum conyzoides, Galinsoga parviflora, Crassocephalum crepidioides, Calyptocarpus vialis, Sonchus arvensis, Eclipta prostrata, Synedrella nodiflora, Tridax procumbens, and Emilia sonchifolia.Argeratum conyzoides L. can be used as a natural herbicide to control weeds in rice fields. A total of 2.0 t ha-1 of Agerattum conyzoides leaf powder applied for two days can reduce 75% growth of rice weeds. In addition, there are also reported three phenolic compounds identified in leaves, stems and roots. These phenolic compounds are gallic acid, caumalic acid, protocatechuic acid [42]. Another benefit of Ageratum conyzoides is usually used as traditional medicine, in Central Africa this plant is used to treat pneumia, wounds, burns [25].Leaf extract from Galinsoga parviflora has the ability to inhibit germination and shoot growth from rice, soybeans, and corn although the inhibition percentage is only about 3.4%. The effect of leaf extract from Galinsoga parviflora is more effective in inhibiting root growth, with an inhibition percentage of > 20% [8]. Based on this, Galinsoga parviflora has the potential as a herbicide.Calyptocarpus vialis Less. have allelopathic properties that can be used as natural herbicides. It is proven that extracts from the roots, stems and leaves of Calyptocarpus vialis were able to inhibit seed germination from Bidens pilosa and Synedrella nodiflora. The higher extract concentrations (4%, 6%, 8%, 10% and 12%) will provide a stronger inhibitory effect, even there are only one or two seeds (total of 10 seeds) that have radicles (would be roots) and the size is very short [32].The compound content found in Calyptocarpus vialis Less. in the form of phenols, tannins, saponins, glycosides, steroids, terpenoids, and coumarin [22].Sonchus arvensis L. has allelopathic properties that can inhibit the growth of other plants, this has been proven in studies using corn kernels. The results of this study indicate that extracts from the plant part of Sonchus arvensis L. (leaves and stems) can inhibit the growth of corn kernels. Based on the analysis, leaf extract contains four kinds of phenolic compounds namely quercetin, hydrogenic acid, ferulic acid and coumaric acid, while the stem extract contains two phenolics namely quercetin and vanillic acid [5], because of the allelopathic activity of these plants it can concluded that Sonchus arvensis L. has the potential as a herbicide.Crassocephalum crepidioides (Benth.) S.More. have allelopathic activity, this is evidenced by studies using leaf extract of Crassocephalum crepidioides (Benth.) S.More. The results showed that leaf extract with a concentration of 25 0.25 gmL-1 can inhibit the growth of Amaranthus retroflexus L., Echinochloa crusgalli (L.) Beauv. and Digitaria sanguinalis (L.) Scop [39]. So it can be concluded that this plant has the potential as a herbicide.Extracts from Eclipta prostrata (L.) L. have potential as herbicides. This was evidenced in studies using Amaranthus spinosus L., Cassia tora L. and Cassia sophera L. which were given leaf extract from Eclipta prostrata (L.) L. The results showed a growth inhibition on the three test plants [17] According to [30] regarding the allelopathic activity of Eclipta prostrata (L.) L. shown in the research carried out on Medical Melilotus alba, with the provision of leaf extract, stem and root of Eclipta prostrata (L.) L. The result is germination from Medicinal Melilotus. inhibited even the radicles or prospective roots are also stunted by growth.Sinedrella nodiflora contains flavonoids, alkaloids, glycosides, steroids, tannins, saponins, phytosterols and triterpenoids. [2]. Some of the compounds contained are groups of allelochemical groups that have allelopathic properties. Leaf extract from Synedrella nodiflora (L.) Gaertn., can inhibit germination and seed growth from tomato plants. This is evident when tomato seeds are given extract from the leaves of Synedrella nodiflora (L.) Gaertn. Seeds that have germination of about 35% of the total whole with a given solution concentration of 20%. Based on this, Synedrella nodiflora (L.) Gaertn. has the potential as an herbicide [16].Tridax procumbens L. has allelopathic properties that can be used as a herbicide. Plants experiment (Vigna radiata L., Dolichos biflorus L. and Vigna unguiculata L.) given leaf extract from Tridax procumbens L. experienced germination inhibition, the higher the extract concentration (25%, 50% and 75%) given the percentage of germination getting smaller. In addition, inhibition also occurs in the shoots and root length, even at a concentration of 75% of the leaf extract can reduce the root length of about 3.4 cm and shoot length of 4 cm when compared with the control [33], so it can be concluded that Tridax procumbens has the potential as a herbicide.Seeds (Zea mays, Citrullus vulgaris, Abelmoschus esculentus, Vigna unguiculata, Glycine soja, and Arachis hypogea) given leaf extract from Emilia sonchifolia showed germination inhibition, even if the extract concentration was increased (0.2, 0.4 and 12 Mg ha-1) almost all the percentage of seed germination decreased [38]. The results of phytochemical screening showed that the leaves of Emilia sonchifolia contained tannins, triterpenoids, saponins, anthraquinones, flavonoids and alkaloids, where several of these compounds were included in the allelochemical group [13]. Based on these results Emilia sonchifolia has the potential as a herbicides.
4. Conclusions
- Asteraceae has 13 the potential as a natural herbicide, namely Argeratum conyzoides, Galinsoga parviflora, Crassocephalum crepidioides, Calyptocarpus vialis, Sonchus arvensis, Eclipta prostrata, Synedrella nodiflora, Tridax procumbens, and Emilia sonchifolia. Four types have not been revealed about allelopathic activity which can be used as an herbicide. Distinctive character from Argeratum conyzoides, flowers do not have ligula, the type of inflorescence is the capitulum. Galinsoga parviflora, an acute apical leaf, forms of laceolate leaves. Crassocephalum crepidioides, flower tube in brownish red color. Calyptocarpus vialis have small stems, rough stem surfaces. Sonchus arvensis has the color of yellow flowers, leaves length 9 - 33 cm. Tridax procumbens, the shape of the stem is procumbent. Emilia sonchifolia, leaf base is auriculate, leaf attachment is decurrent. Youngia japonica, apical leaf is obtuse, yellow flower has ligule. Eclipta prostrata, the number of stamen is 4, the style is bifid. Acmella paniculata, leaf color is thick dark green, slippery leaf surface. Synedrella nodiflora, the shape of the stem is tetragonal. Vernonia cinerea, leaf arrangement is dextrorse. Senecio vulgaris, leaf arrangement is cauline.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML