-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Basic Sciences of Medicine
p-ISSN: 2167-7344 e-ISSN: 2167-7352
2018; 7(1): 1-6
doi:10.5923/j.medicine.20180701.01

Effect of Prolonged Administration of Artemether-Lumefantrine on Testicular Biomarkers of Oxidative Stress: Ameliorative Effect of Vitamin E
Onuche A. Daikwo 1, Mohammed U. Kawu 2, Rabiu A. Magaji 1, Ejike D. Eze 3
1Department of Human Physiology, Faculty of Medicine, Ahmadu Bello University, Zaria, Nigeria
2Department of Physiology, Faculty of Veterinary Medicine, Ahmadu Bello, University, Zaria, Nigeria
3Department of Physiology, Faculty of Biomedical Sciences, Kampala International University, Ishaka-Bushenyi, Uganda
Correspondence to: Onuche A. Daikwo , Department of Human Physiology, Faculty of Medicine, Ahmadu Bello University, Zaria, Nigeria.
| Email: |  |
Copyright © 2018 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Recurrence of malaria infection in Nigeria encourages frequent use of artemisinin-based combination therapies (ACTs). The ACTs function via generation of free radicals. Reactive oxygen species have been implicated in the etiology of male infertility. The present study evaluated prolonged administration of artemether-lumefantrine (AL) and the ameliorative effect of vitamin E (VE) on testicular biomarkers of oxidative stress. Adult male Wistar rats (n = 35) were divided into five groups of seven rats each and treated twice daily for a period of 2 weeks via oral route as follows; group I: 1ml/kg distilled water; group II: 1ml/kg olive oil; group III: 2.29 mg/13.74 mg/kg AL; group IV: 2.29 mg/13.74 mg/kg AL + 100 mg/kg of VE; group V: 2.29 mg/13.74 mg/kg AL + two weeks recovery (allowed to recover from AL treatment for 2 weeks before sampling). At the end of the experiment, animals were sacrificed and testes harvested for analysis of testicular biomarkers of oxidative stress. Testicular concentrations of antioxidant enzymes glutathione peroxidase (GPx) and catalase (CAT) were significantly (P < 0.05) higher in groups IV and V rats when compared with other groups. Testicular malondialdehyde (MDA) level was increased, while superoxide dismutase (SOD) was decreased in group III as compared to other groups, though these changes were not significant. It is concluded that, prolonged administration of artemether-lumefantrine induces significant alterations in testicular antioxidant enzymes activity suggestive of oxidative stress and that vitamin E and cessation of treatment with artemether-lumefantrine appreciably ameliorates the alterations in testicular antioxidant enzymes activity.
Keywords: Artemether-lumefantrine, Oxidative stress, Testes, Vitamin E
Cite this paper: Onuche A. Daikwo , Mohammed U. Kawu , Rabiu A. Magaji , Ejike D. Eze , Effect of Prolonged Administration of Artemether-Lumefantrine on Testicular Biomarkers of Oxidative Stress: Ameliorative Effect of Vitamin E, Basic Sciences of Medicine , Vol. 7 No. 1, 2018, pp. 1-6. doi: 10.5923/j.medicine.20180701.01.
Article Outline
1. Introduction
- Infertility, a major medical and social predicament, is defined as the inability to conceive after at least, twelve months of regular and unprotected intercourse by couples of reproductive age. It occurs when a man cannot impregnate a proven fertile female or when a woman does not conceive after one year of unprotected intercourse with a proven fertile male. In time past, most of the blame for infertility was placed on the female. However, considerable attention is now being given to the male factor. [1, 2]. In Sub-Saharan Africa, the prevalence of infertility is as high as 30% and the male contribution in most countries including Nigeria had been variously estimated to be between 25 and 50% [3-5].Recurrence of malaria (caused by either a recrudescence; a relapse or a new infection; [6], has resulted in the uncontrolled use of artemisinin-based combination therapies (ACTs) in endemic areas of the disease, such as Nigeria. Moreover, self-medication and purchase of anti-malaria drugs in the open market is rampant [7]. Artemisinins target malaria-infected erythrocytes and destroy the parasites through generation of free radicals [8, 9].Oxidative stress mediated by reactive oxygen species (ROS) is being recognized more commonly as a causative factor in male infertility [10]. In males, the developing germ cells, spermatogonia and spermatocytes of the testis are highly sensitive and undergo apoptosis in conditions that cause oxidative stress [11]. This vulnerability to oxidative insult ensues from the high lipid content of the testis.Vitamin E is a fat-soluble antioxidant and protects cell membranes that are mainly composed of fatty acids [12].The present study evaluated prolonged administration of artemether-lumefantrine (coartem) and the ameliorative effect of vitamin E (VE) on testicular biomarkers of oxidative stress.
2. Materials and Methods
2.1. Experimental Animals
- Thirty-five (35) male Wistar rats (130–150 g) obtained from the animal house of the Department of Pharmacology and Therapeutics, Ahmadu Bello University Zaria, Nigeria were used for the study. They were kept in rat cages in the Animal House of the Department of Human Physiology, Ahmadu Bello University, Zaria. They had free access to food and water and allowed to acclimatise for a period of two weeks before the commencement of the experiment.
2.2. Animals Grouping and Dosage of Administration
- The thirty-five (n = 35) male Wistar rats used for the study were divided into five groups of seven (n = 7) rats each and treated as follows:Group I: Each rat was administered with distilled water at 1ml/kg body weight Group II: Each rat was administered with olive oil at 1ml/kg body weight Group III: Each rat was administered with 2.29 mg/13.74 mg/kg Artemether/Lumefantrine dissolved in distilled water.Group IV: Each rat was administered with 2.29 mg/13.74 mg/kg Artemether/Lumefantrine + 100 mg/kg of Vitamin E dissolved in olive oil.Group V: Each rat was administered with 2.29 mg/13.74 mg/kg Artemether/Lumefantrine and subsequently allowed to recover for 2 weeks during which no treatment was given.The dose of Artemether/Lumefantrine (Coartem
 ) used for this study was calculated from the manufacturer's recommended dose for a man weighing at least 35 kg, a modification of Otuechere et al. [13].
) used for this study was calculated from the manufacturer's recommended dose for a man weighing at least 35 kg, a modification of Otuechere et al. [13]. 2.3. Drugs and Chemicals
- The Coartem (Artemeter/lumefantrine: 20 mg/120 mg; Novartis Pharmaceuticals Corporation – Switzerland), Vitamin E (α-tocopherol; Gujarat Liqui Pharmacaps Pvt. Ltd. – India: NAFDAC Reg. No. A4-8322) and olive oil used for this study were purchased from a reputable pharmaceutical store in Zaria, Kaduna State, Nigeria. The Vitamin E (1000 mg) was aspirated into a syringe and then reconstituted with olive oil (vehicle) prior to daily administration.
2.4. Drug Preparation and Administration
- The coartem was ground to a powdered form, mixed with distilled water and administered as an aqueous suspension. The drug suspension was continuously agitated during administration in order to deliver the drug homogeneously to the animals. Vitamin E was aspirated using a 1 ml syringe and reconstituted in olive oil. The respective treatments were administered to all rats in all groups twice daily for a period of two weeks using a 1.0 mL syringe by oral gavage. However, rats in group V were allowed to recover for 2 weeks after the last treatment before they were sacrificed.
2.5. Sample Collection
- At the end of the treatment and recovery period (group V), all rats were anaesthetised by chloroform inhalation in a closed chamber and thereafter, sacrificed. The testis was carefully removed and homogenised in a physiological solution (7.4 pH phosphate buffered saline) and the homogenate centrifuged at 1788 × g for 10 mins. The supernatant was removed and used for assessment of testicular biomarkers of oxidative stress.
2.6. Assay of Testicular Biomarkers of Oxidative Stress
- Assay of biomarkers of oxidative stress namely; lipid peroxidation (malondialdehyde); antioxidant enzymes (superoxide dismutase, glutathione peroxidase and catalase) was based on the Enzyme-linked immunosorbent assay (ELISA) technique, using their respective ELISA kits (WKEA MED SUPPLIES CORP). The manufacturer's manuals were strictly followed.
2.7. Statistical Analysis
- SPSS version 17 was used for the statistical analysis. The data obtained were calculated by one-way analysis of variance (ANOVA) and compared using the Tukey's post hoc test. Differences were considered statistically significant at P <0.05.
3. Results
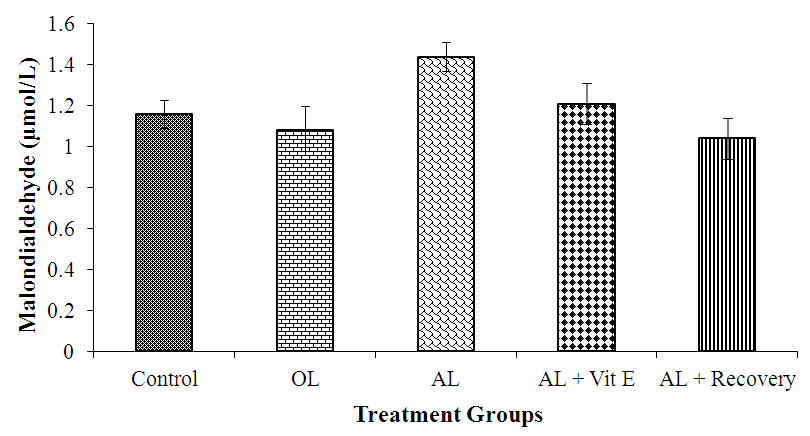
3.1. Effect of Prolonged Administration of Artemether-Lumefantrine on Testicular Malondialdehyde (MDA) Concentration
- The effect of prolonged administration of artemether-lumefantrine on intra-testicular malondialdehyde (MDA) concentration is shown in figure 1. Testicular MDA levels were higher in rats treated with AL only (group III) than in other groups. While, testicular MDA (μmol/L) concentration was lowest in the AL + Recovery group as follows: AL + Recovery, 1.04 ± 0.10 vs control, 1.61 ± 0.07; OL, 1.08 ± 0.12; AL, 1.44 ± 0.07; AL + Vit E, 1.21 ± 0.10, respectively. However, the differences in MDA concentrations between the groups were not statistically significant.
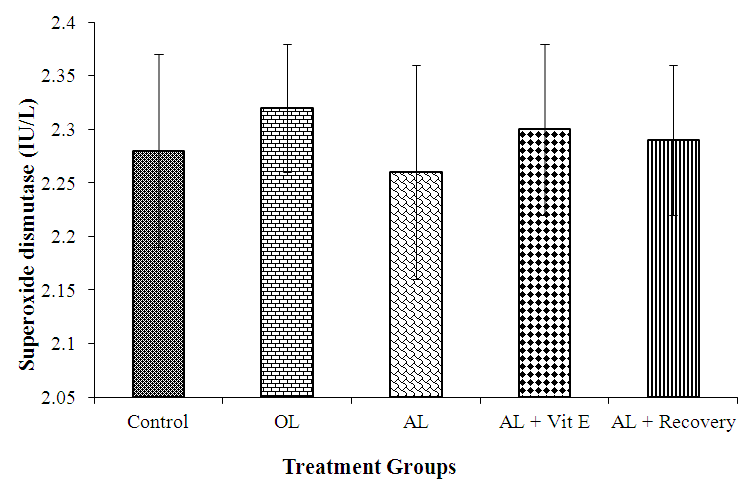
3.2. Effect of Prolonged Administration of Artemether-Lumefantrine on Testicular Concentrations of Superoxide Dismutase (SOD)
- The effect of prolonged administration of artemether-lumefantrine on testicular concentrations of SOD is shown in figure 2. Testicular SOD concentration (IU/L) for the five groups were as follows: control, 2.28. ± 0.09; OL, 2.32. ± 0.60; AL, 2.26 ± 0.10; AL + Vit E, 2.30 ± 0.08 and AL + Recovery, 2.29 ± 0.07, respectively. There was no statistical difference in testicular SOD concentration between the groups.
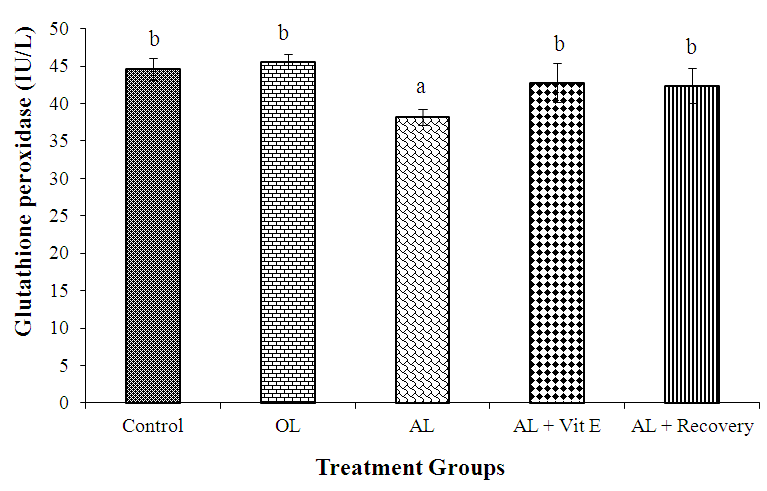
3.3. Effect of Prolonged Administration of Artemether-Lumefantrine on Testicular Concentrations of Glutathione Peroxidase (GPx)
- The effect of prolonged administration of artemether-lumefantrine on testicular concentration of glutathione peroxidase (GPx) is presented in figure 3. The testicular concentration of GPx (IU/L) was significantly (P < 0.05) lower in rats treated with AL only (group III) than in other groups (AL: 38.20. ± 1.11 vs control: 44.6 ± 1.44; OL: 45.6 ± 1.03; AL + Vit E: 42.80 ± 2.63 and AL + Recovery: 42.4 ± 3.32, respectively; P < 0.05).
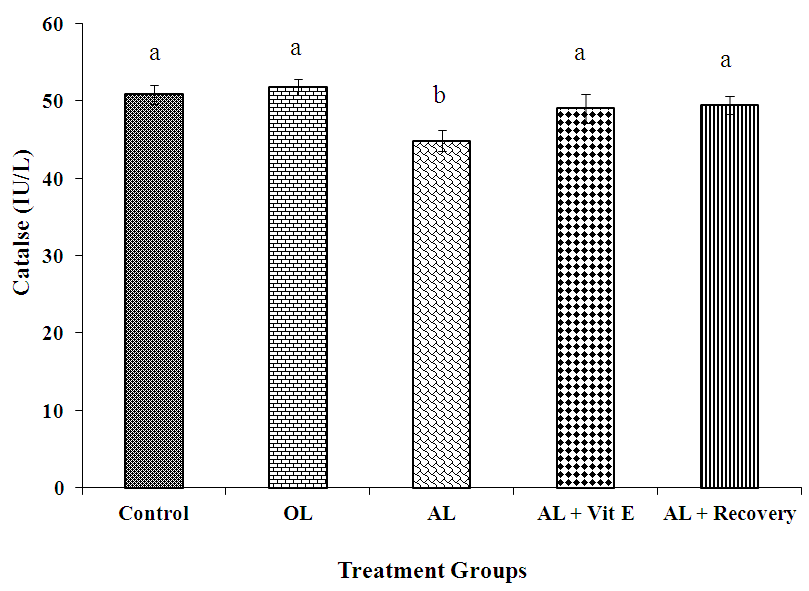
3.4. Effect of Prolonged Administration of Artemether-Lumefantrine on Testicular Catalase Concentrations
- The effect of prolonged administration of artemether-lumefantrine on testicular concentrations of catalase (CAT) is presented in figure 4. Rats treated with AL only had significantly (P < 0.05) lower testicular catalase (IU/L) concentration than other groups (AL: 44.80. ± 1.36 vs control: 50.80 ± 1.24; OL: 51.80 ± 1.02; AL + Vit E: 49.00 ± 1.87 and AL + Recovery: 49.40 ± 1.17, respectively).
4. Discussion
- In the present study, rats were treated with 2.29 mg/13.74 mg/kg artemether/lumefantrine (AL) for two weeks. The choice of dose and route of administration was done as recommended for humans, but the 2 weeks duration of treatment was to expose the animals to treatment beyond the recommended duration, to mimic the extended duration of treatment associated with recurrence of malaria due to incomplete treatment or endemicity.The testis contains an array of antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPx). A balance normally exists between free radicals (oxidants) production and these antioxidant scavenging enzymes activities. As a result of such balance, only physiological amounts of oxidants remain, which are needed for the regulation of normal sperm functions, such as sperm capacitation, hyper-activation, acrosome reaction and sperm–oocyte fusion. The production of excessive amounts of oxidants in the testis can overwhelm the antioxidant defence mechanisms and cause oxidative stress, which decreases enzymatic defences of the testes and cause damage to spermatozoa thereby compromising sperm quality and functions [14]. In the present study, an insignificant increase in level of malondialdehyde (MDA), a marker of lipid peroxidation was observed in the AL group. The reason for this is not far-fetched, because anti-malarial drugs owe their efficacy to their oxidant effect and artemether-lumefantrine in particular, generates free radicals due to presence of an endoperoxide bridge [15, 16]. This finding agrees with an earlier report of increased MDA concentration observed in artesunate-treated rats [17]. Similarly, significantly lower levels of the antioxidant enzymes, glutathione peroxidase (GPx) and catalase (CAT), but insignificantly lower concentration of superoxide dismutase (SOD) was observed in the AL group compared with other groups. Superoxide dismutase is considered the first line of defence against deleterious effects of oxygen radicals in cells by catalyzing the dismutation of superoxide anion (O2.-) to hydrogen peroxide (H2O2) and molecular oxygen (O2). This antioxidant enzyme is present in an essential amount in the germinal cells of the testis wherein constant cell division occurs during spermatogenesis [11]. This suggests that perhaps, the testis is highly sensitive to superoxide radicals (O2.-) constantly produced during spermatogenesis. Hence, the SOD immediately dismutates O2.- to yield H2O2 and molecular oxygen (O2). The testis now suffers the ill-effect of the eventual generation of H2O2 which is supposedly acted upon by CAT and GPx. However, Lysiak and Turner [11], reported that the level and activities of these enzymes (CAT and GPx) within the germinal cells are not sufficient enough to quickly buffer the continuously generated H2O2 and other free radicals. This could be responsible for the significant reduction in the levels of these two enzymes in the AL group, as their levels perhaps, constantly decline in an attempt to neutralise the effect of H2O2. Similar findings of significant reduction in the levels of antioxidant enzymes in the testis of animals exposed to anti-malaria agents have been reported [16, 18, 19].The Vitamin E treated and recovery groups gave promising results as most of the parameters investigated in this study were comparable to the baseline levels obtained in the control group. A remarkable but insignificant decrease in malondialdehyde and an increase in antioxidant enzymes were observed in these groups. Although results in these groups were similar to the baseline levels obtained in the control group, it should be noted that animals in control group were not administered artemether-lumefantrine. This may suggest that Vitamin E assumed its antioxidant function, putting the endogenous antioxidant enzymes on hold, thereby buffering the negative effect of free radicals generated by artemether-lumefantrine as indicated by the lower level of malondialdehyde in the A/L + Vit E group as compared to the control group.Vitamin E (α-tocopherol) is a naturally occurring antioxidant that plays an important role by inactivating harmful free radicals produced through normal cellular activity and from various stressors. It terminates lipid peroxidation and stabilizes the molecular composition of cellular membranes, thus, preventing the harmful effects of reactive oxygen species (ROS) [20-22]. Restoration to normalcy observed in the recovery group of this study suggests that the deleterious effects of artemether-lumefantrine on the parameters studied could be spontaneously corrected with time after cessation of administration.
5. Conclusions
- Based on the findings of this study, it is concluded that prolonged administration of artemether-lumefantrine in male Wistar rats induces significant alterations in some testicular antioxidant enzymes activity, suggesting oxidative stress, and, that vitamin E and ceasation of treatment with artemether-lumefantrine causes appreciable amelioration of the alterations in testicular biomarkers of oxidative stress.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML