-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Basic Sciences of Medicine
p-ISSN: 2167-7344 e-ISSN: 2167-7352
2017; 6(1): 4-13
doi:10.5923/j.medicine.20170601.02

The Preventive Effects of Avocado Fruit and Seed Extracts on Cardio-nephrotoxicity Induced by Diethylnitrosamine/2-acetylaminoflurine in Wistar Rats
Adel Abdel-Moneim A. , Osama M. Ahmed , Hanaa I. Fahim , Eman E. Mohamed
Molecular Physiology Division, Zoology Department, Faculty of Science, Beni-Suef University, Beni-Suef, Egypt
Correspondence to: Adel Abdel-Moneim A. , Molecular Physiology Division, Zoology Department, Faculty of Science, Beni-Suef University, Beni-Suef, Egypt.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The current study was designed to evaluate the possible preventive effects of avocado (Persea americana) fruit and seed hydroethanolic extracts on diethylnitrosamine/2-acetylaminoflurine (DEN/2AAF)-induced cardio-nephrotoxicity in Wistar rats. The Wistar rats used in this study were allocated into 4 groups. Group I was kept as a normal group, while the other 3 groups were administered a twice intraperitoneal dose (150 mg/kg bw./week) of DEN, followed by daily 2AAF-administration by oral gavage four days per week for three weeks. Group II was DEN/2AAF-administered control group and groups III and IV were administered DEN/2AAF and were orally given avocado fruit and seed hydroethanolic extract for 20 weeks. DEN/2AAF-administration induced kidney and heart injury evidenced by histological alterations as well as significant increase of serum indicators of impaired kidney function including creatinine, urea and uric acid levels and serum marker of impaired heart function including CK, CK-MB, LDH and AST activities. On the other hand, the renal and cardiac lipid peroxidation was elevated while renal and cardiac glutathione content and activities of superoxide dismutase, glutathione peroxidase and glutathione-S-transferase were significantly declined as a result of DEN/2AAF-administrations. Concomitant supplementation with avocado fruit and seed hydroethanolic extract potentially prevented DEN/2AAF-induced biochemical and histopathological deleterious alterations. In conclusion, the results suggest that avocado fruit and seed hydroethanolic extracts successfully have ameliorative effects against DEN/2AAF-induced cardio-nephrotoxicity via the enhancement of the antioxidant defense system and suppression of the inflammatory action.
Keywords: Persea americana, Diethylnitrosamine, Acetylaminofluorene, Cardio-nephrotoxicity, Antioxidant defence system, Anti-Inflammatory effects
Cite this paper: Adel Abdel-Moneim A. , Osama M. Ahmed , Hanaa I. Fahim , Eman E. Mohamed , The Preventive Effects of Avocado Fruit and Seed Extracts on Cardio-nephrotoxicity Induced by Diethylnitrosamine/2-acetylaminoflurine in Wistar Rats, Basic Sciences of Medicine , Vol. 6 No. 1, 2017, pp. 4-13. doi: 10.5923/j.medicine.20170601.02.
Article Outline
1. Introduction
- Diethylnitrosamine (DEN) is a representative chemical compound of a family of carcinogenic n-nitroso compounds [1]. It has been found that DEN distributed in many processed food including meats, tobacco smoke, whisky, cheese, cured meat and alcoholic beverages [2]. Oxidative stress is defined as an imbalance between the generation and elimination of reactive oxygen species (ROS) [3]. ROS have been implicated in the mechanisms that lead to tubular necrosis and play a key role in the pathogenesis of drug-induced renal damage [4]. The high levels ROS are involved in the initiation and progression of different types of cardiovascular diseases [5].Flavonoids and phenolic compounds possess antioxidant activity which have free radical scavenging mechanism and produce potential alteration of physiological antioxidant status and imbalance in the free radical defence enzymatic system [6]. The biological activities of the flavonoids are related to their antioxidant activity by various mechanisms, e.g. by scavenging or quenching free radicals, by inhibiting enzymatic systems responsible for the generation of free radicals or by chelating metal ions [7]. Vegetables and fruits are essential foods in our diet and also have many compounds that are beneficial for health due to minor components, these minor components include phenolic substances [8].The fruit of avocado (Persea Americana), commonly known as avocado is an edible fruit from Central America which is easily adaptable in tropical regions [9]. Avocado is considered the world’s healthiest fruit, because of its nutrient contents such as vitamin K, dietary fiber, potassium, folic acid, vitamin B6, vitamin C, copper and reasonable calories. It is one of the most recommended fruits as well as a food for body building and medicine for cholesterol-related diseases and it was found to have high cholesterol levels [10].The avocado seed represents 16% of total avocado weight and is an under-utilized resource [11]. Phytochemical studies on avocado seeds have identified various classes of natural compounds such as phytosterols, triterpenes, fatty acids, furanoic acids, abscisic acid, proanthocyanidins, and polyphenols [12, 9]. Therefore, this study is designed to assess the preventive effects of avocado fruit and seed hydroethanolic extract on DEN/2AAF-induced kidney and heart toxicity in male Wistar rats.
2. Materials and Methods
2.1. Experimental Animals
- Adult male Wistar rats, weighing 100-120 g, were used in the present study. The animals were obtained from Animal House Facility of Egyptian Organization for Biological Products and Vaccines (VACSERA), Helwan, Cairo. They were kept under observation for two weeks before the onset of the experiment to exclude any intercurrent infections. The animals were housed in polypropylene cages with good aerated stainless steel covers in the Zoology department Animal House in Faculty of Science, Beni-Suef University, Egypt at normal temperature (20-25°C) and normal daily lighting cycle (10-12 hr/day), and given enough water and food (balanced standard diet). All animal procedures are in accordance with the general guidelines of animal care and the recommendations of the Experimental Animal Ethics Committee of Faculty of Science, Beni-Suef University, Egypt.
2.2. Chemicals
- Diethylnitrosamine (DEN) and, 2- acetylaminoflourine (2AAF), was purchased from Sigma Chemicals Co., St. Louis, MO, USA, stored at 2-4°C sunlight. All other chemicals used for the investigation were of analytical grade.
2.3. Preparation of the Avocado Fruit and Seed Extracts
- Avocado pear (Persea americana) fruit was purchased from the local market in Beni-Suef, Egypt and was authenticated by Dr. Mohamed Ahmed Fadl, lecturer of taxonomy, Botany Department, Faculty of Science, Beni-Suef University, Egypt. Avocado (fruit and seed) were extracted according to the method of Biglari et al. [13]. This method was modified to obtain the maximum yield. Fruits and seed were separately pitted, cut into small pieces, dried in a well aerated shade area for 15 days, coarsely powdered and then were extracted with 1 liter of 70% ethanol (1:2 w/v), at room temperature (25°C) for 72 hours. The extracts were then filtered through Whatman filter paper and concentrated under reduced pressure, using a rotary evaporator to obtain the hydroethanolic crude extracts of the fruits and seeds. The yield was found to be 1%. The extracts were kept in dark glass bottles and stored at-18°C until used.
2.4. Induction of Cardio-nephrotoxicity
- Cardio-nephrotoxicity was induced by intraperitoneal injection of two doses of DEN (150 mg/kg bw./ week) dissolved in 0.9% saline at the beginning of experiment for two weeks followed by weekly administration of 2AAF (20 mg/kg bw.), in 1 % tween 80, by oral gavage four days per week for three weeks [14], then rats were sacrificed after 20 weeks from the beginning of the experiment.
2.5. Experimental Design
- The experiment was performed on forty adult male Wistar rats which were randomly distributed into 4 groups, each of ten animals. The 1st group was kept as a normal group, while the other 3 groups were administered DEN, followed by 2AAF as previously described. One of three DEN/2AAF-administered groups were kept as control while the two others were orally given avocado fruit and seed hydroethanolic extract every other day for 20 weeks. Avocado fruit and seed hydroethanolic extract at a dose of 50 mg/kg bw. [15] dissolved in 1% carboxymethyl cellulose (CMC), every other day till the end of the experiment for 20 weeks. By the end of the experiment, animals were sacrificed under mild anaesthesia. Blood samples were collected from jugular veins, left to coagulate and centrifuged at 3000 rpm for 15 min to separate the serum. Kidney samples were immediately excised and perfused with ice-cold saline. Frozen kidney samples were homogenized in chilled saline (10% wt/vol) by using Telfon homogenizer (Glas-Col, Terre Haute, USA) and the homogenates were centrifuged at 3000 rpm for 10 min. The homogenate supernatants were collected and kept in a deep freezer at –30°C until used for the determination of oxidative stress parameters and antioxidant defence markers.
2.6. Biochemical Investigations
- Serum creatinine, urea and uric acid levels were determined by using kits obtained from Biosystem S.A. (Spain) according to the manufacturer's protocol. Serum total CK activity was determined by using reagent kit obtained from VITRO (Germany). Serum CK-MB and LDH were determined by using kits obtained from HUMAN (Germany). Serum AST activity was determined according to the reagent kits purchased from Biosystem S.A. (Spain). The supernatants were used for estimation of lipid peroxidation (LPO) according to the method of Yagi [16] by using chemical reagents prepared in the laboratory. Reduced glutathione (GSH) was assayed according to the method of Beutler et al. [17]. Activities of kidney and heart antioxidant enzymes including superoxide dismutase (SOD), glutathione peroxidase (GPx), and glutathione-S-transferase (GST) were measured according to the methods of Marklund and Marklund [18], Matkovics et al. [19] and Mannervik and Gutenberg [20] respectively by using chemical reagents prepared in laboratory.
2.7. Histopathological Investigation
- Kidneys and hearts from each animal were excised after dissection. One kidney was fixed in neutral buffered formalin for 24 hrs and then transferred into 70% alcohol for histopathological examination. Paraffin wax tissue blocks were prepared for sectioning at 4 microns thickness by slide microtome. The obtained tissue sections were mounted on glass slides, deparaffinised, stained with haematoxylin and eosin (H&E) stain [21]. The examination was done through the light electric microscope.
2.8. Statistical Analysis
- Results were expressed as mean ± standard error (SE). The data were analysed Duncan′s method for post-hoc analysis (SPSS version 20 software) was used to compare other data between different groups with significance set at P<0.05.
3. Results
3.1. Biochemical Effects
- With regard to the kidney and heart function the administration of DEN/2AAF to Wistar rats for 20 weeks produce a significant elevation (p<0.05) in urea, uric acid, CK-MB, LDH and AST levels as compared to the normal group. The treatment of DEN/2AAF-administered rats with avocado fruit and seed hydroethanolic extract produced a significant amelioration (P<0.05) of the elevated serum urea, uric acid, CK-MB, LDH and AST levels as compared to DEN/2AAF-administered control group (Table 1).
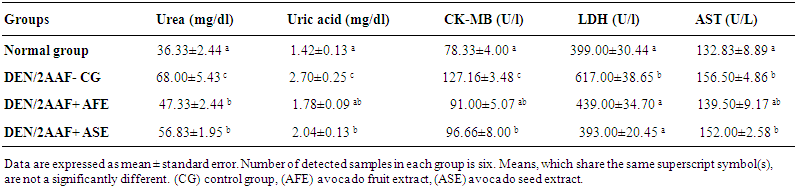 | Table 1. Effect of avocado fruit and seed extracts on serum urea and uric acid concentrations and CK-MB, LDH and AST activities in DEN/2AAF administered rats |
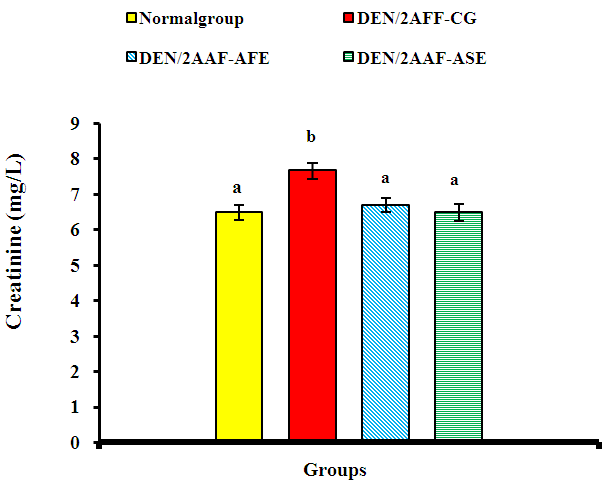 | Figure 1. Effect of avocado fruit and seed extracts on serum creatinine level in DEN/2AAF-administered rats |
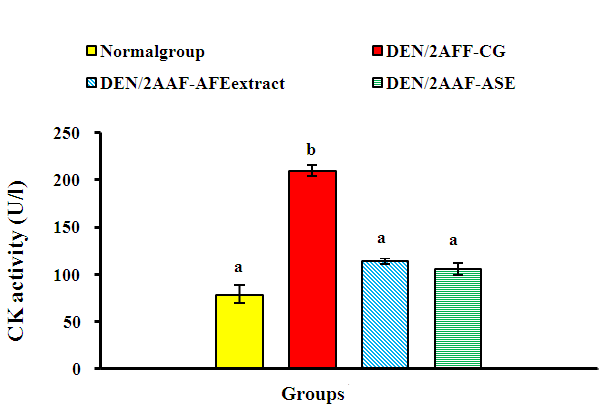 | Figure 2. Effect of avocado fruit and seed extracts on serum CK activity in DEN/2AAF-administered rats |
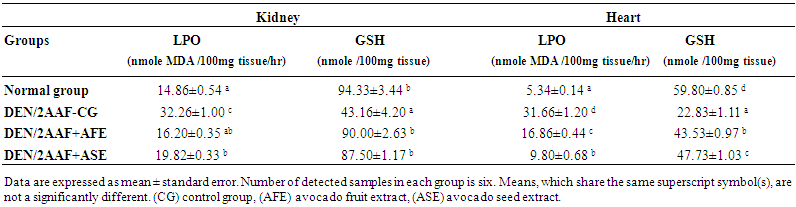 | Table 2. Effect of avocado fruit and seed extracts on kidney and heart LPO and GSH levels in DEN/2AAF administered rats |
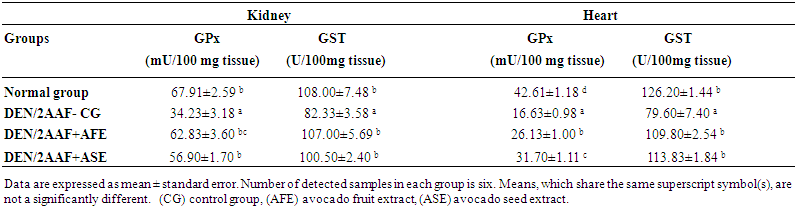 | Table 3. Effect of avocado fruit and seed extracts on kidney and heart; GPx, GST and SOD activities in DEN/2AAF-administered rats |
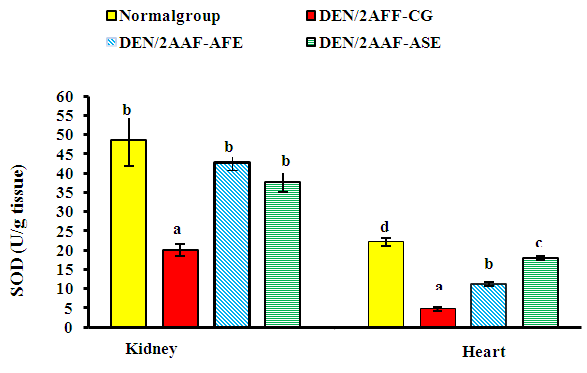 | Figure 3. Effect of avocado fruit and seed extracts on kidney and heart SOD activity in DEN/2AAF-administered rats |
3.2. Effect on Kidney and Heart Histological Changes
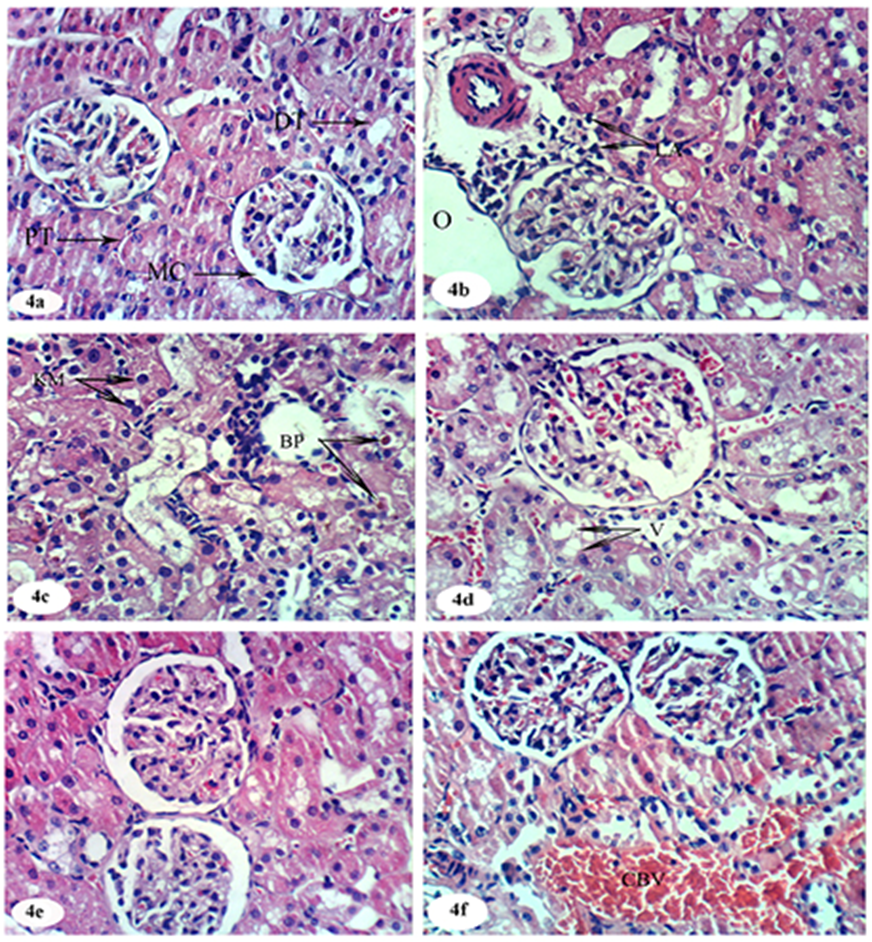
- Histopathological examination revealed normal histology of kidney in the normal group (Figure 4a). The DEN/2AAF-administration produced histopathological changes in the kidney like mononuclear leucocytic aggregation and perivascular oedema, severe vacuolar degenerative changes of the epithelial lining of renal tubules, brown pigments accumulation and few karyomegalic nuclei (Figure 4b, 4c and 4d). On the other hand, the treatments of DEN/2AAF with avocado fruit hydroethanolic extract showing nearly normal structure (Figure 4e). The treatment of DEN/2AAF-administered rats with avocado seed hydroethanolic extract showed congestion in the blood vessels with nearly normal histological structure (Figure 4f).
4. Discussion
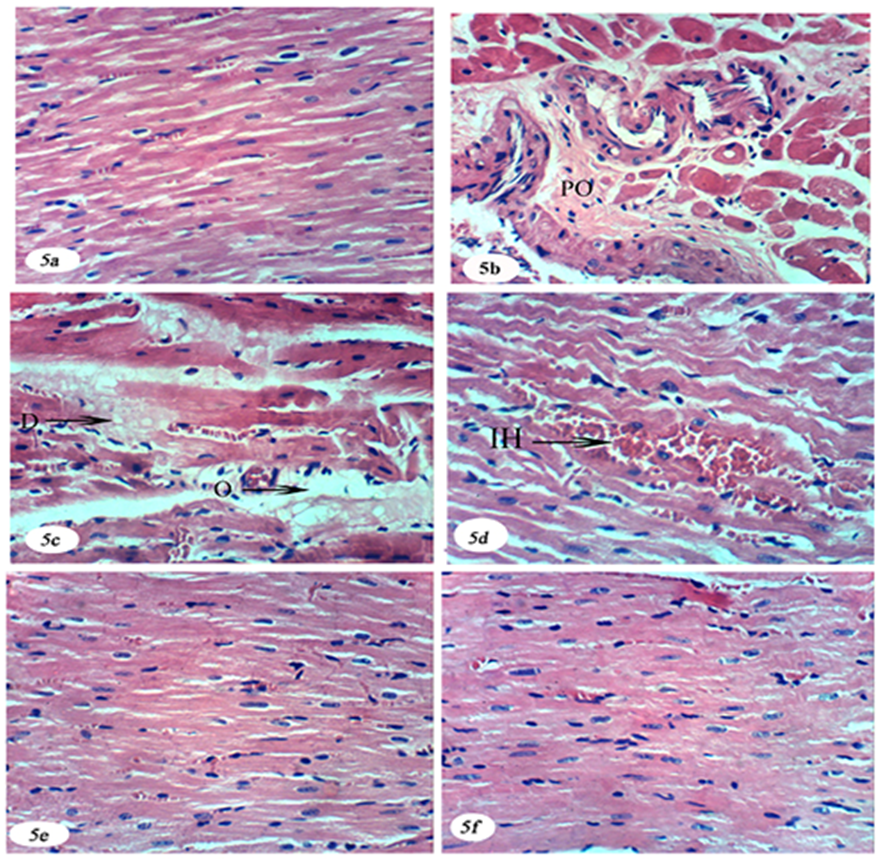
- The kidney is an essential excretory organ of the body because it plays a dominant role in homeostasis by excreting the metabolic waste products and excess necessary substances. Metabolites of the drugs that are excreted by kidney may also cause cellular damage leading to kidney dysfunction. Several xenobiotics exert their toxic effects by one or more common pathogenic mechanisms that can produce nephrotoxicity [22]. The present study showed that the administration of DEN/2AAF- induced renal dysfunction, as evidenced by the increased levels of serum creatinine, urea, and uric acid as compared to the normal group. These findings are in agreement with the studies of Ahmed et al. [23] and Abdel-Moneim et al. [24], who reported that administration of DEN increased serum levels of creatinine, urea and uric acid.Serum creatinine level relates to glomerular function and its rise is an indicator of renal failure [25]. Diethylnitrosamine administration to rats lead to a marked elevation in the levels of serum urea which is indicative of kidney damage, as previously reported by various publications [26]. In addition, Abdel-Moneim et al. [24] demonstrated increased serum urea and creatinine levels in DEN-administered rats. The elevation of uric acid in the blood (hyperuricemia) is considered as a sensitive marker of inflammation taking place at various sites of the body [27]. A high serum uric acid level can be derived from renal dysfunction in excretion of serum uric acid [28] or as a result of increased cell apoptosis and necrosis [29].On the other hand, the DEN/2AAF-administered groups treated with avocado fruit and seed hydroethanolic extract improved the kidney functions as evidenced by the reduction of the elevated serum creatinine, urea and uric acid concentrations. These findings are in agreement with the studies of Mahadeva et al. [30] who reported that avocado fruit acts as nephro-protective agent. Avocado oil-rich diet has been shown to modify the fatty acid content in renal cell membranes [31].Furthermore, administration of avocado fruit extract can significantly decrease the level of uric acid which might be due to the antioxidant property of avocado which prevents the SH group of the enzyme from oxidation and thereby elevated uric acid level [32]. The seeds of avocado (P. Americana) also had protective effects on some rats' tissues such as the kidneys [33] which may, in-turn, improved the deteriorated kidney function parameters in serum of DEN/2AAF-administrated rats treated with seed extract. Cardiotoxicity is a well-known side effect of several cytotoxic drugs, especially of the anthracyclines and can lead to long term morbidity. The mechanism of anthracycline induced cardiotoxicity seems to involve the formation of free radicals leading to oxidative stress. This may cause apoptosis of cardiac cells or immunologic reactions [34]. In the present study, the animals administered DEN/2AAF showed cardiotoxicity and significant increased activities of serum enzymes CK, CK-MB, LDH and AST activities. These results are in agreement with Sheweita et al. [35] who reported that most N-nitrosamines increased CK-MB and LDH activities. It is concluded that N-nitrosamines increased levels of free radicals and decreased the activity of antioxidant enzymes which may consequently increase. High level of diagnostic marker enzymes CK, LDH and AST due to leakage takes place from tissue to blood serum due to damaged or destroyed myocardial cells, because of insufficient supply of oxygen, the cell membrane become fragile or may rupture and the cellular enzymes in serum reflect the alteration in plasma membrane permeability [36]. The increased levels of these enzymes are indicative to a severity of cell necrosis [37]. Myocardium contains plentiful concentrations of diagnostic markers of myocardial infarction such as CK-MB, LDH and AST once metabolically damaged, it releases its contents into the extracellular fluid [38]. LDH is a cytosolic enzyme that catalysis the reversible oxidation of L-lactate to pyruvate; their increased activity in serum confirms increased membrane permeability and cellular leakage [39]. Administration of DEN/2AAF may lead to the damage of the myocardial cell membrane and it became more permeable, that resulted in the leakage of AST, CK, CK-MB and LDH in the blood which probably accounts for the increase in the level of these marker enzymes in the serum. On the other hand, the present study showed that the avocado fruit and seed hydroethanolic extract significantly decreased the heart enzymes CK, CK-MB, LDH and AST in serum. These results are in agreement with Gouegni and Abubakar [40] and Chatterjea and Shinde [41]. The decrease in these enzymes activities in serum as a result of treatment of DEN/2AAF-adminstrated rats with avocado fruit and seed hydro-ethanolic extract reflects that avocado could maintain membrane integrity thereby restricting the leakage of cardiac enzymes [32].The phytochemical compounds in avocado seeds are responsible for prevention and treatment heart disease [42]. The presences of saponin in avocado seed make this fruit more important because saponins have both hypertensive and cardiac depressant properties [43].In addition, the DEN/2AAF-administration produced histopathological changes in the kidney like mononuclear leucocytic aggregation, perivascular oedema, severe vacuolar degenerative changes of the epithelial lining of renal tubules, brown pigments accumulation and few karyomegalic nuclei. The treatments of DEN/2AAF with avocado fruit and seed hydroethanolic extract showing nearly normal renal tubules and congestion in blood vessels. Cardiac injury induced by DEN/2AAF was confirmed by the observed histological alterations that include perivascular oedema, intramuscular oedema, degeneration of certain cardiomyocytes and intramuscular haemorrhage. DEN/2AAF-administered rats treated with avocado fruit and seed extract showing no histopathological changes. These histological changes go parallel with biochemical findings which indicated the improvements of markers of kidney and heart functions in serum after treatment of DEN/2AAF-adminstrated rats with avocado fruit and seed extracts.ROS interact with various tissue compounds leading to dysfunction and injury to the kidney and other organs [2]. The present study showed elevated kidney and heart of LPO in response to the administration of DEN/2AAF in Wistar rats. These results are in concurrence with the studies of Abdel-Moneim et al. [24] and Fukai and Ushio-Fukai [44]. Also, the administered DEN/2AAF showed cardio-toxicity and significant increase of LPO this result may due to excessive production and/or inadequate removal of reactive oxygen species, especially superoxide anion O2− , have been implicated in the pathogenesis of many cardiovascular diseases [44].DEN induced oxidative damage is generally attributed to the formation of highly reactive hydroxyl radical (OH−), a stimulator of LPO and the source of destruction and damage to the cell membrane [45]. DEN increased lipid peroxide formation and cell membrane damage and decreased levels of antiperoxidative enzymes [46]. Excessive generation of oxygen free radicals can cause oxidative damage to biomolecules resulting in lipid peroxidation (LPO), mutagenesis and carcinogenesis. Natural antioxidants may provide a strong defence against the damages of the cellular organelles caused by free radical induced oxidative stress [47].In the present study, GSH content and activity of the antioxidant enzymes GPx, GST and SOD were depleted in kidney and heart in the DEN-2AAF-administered rats. Decrease of kidney GSH content, GPx, GST and SOD activities were in agreement with the studies of Ahmed et al. [48] and Abdel-Moneim et al. [24]. DEN decreases the activities of renal antioxidant enzymes causes depletion in the level of renal glutathione content [49]. Meanwhile, reduction in activity of the antioxidant enzymes in kidney (GSH content GPx, GST and SOD) in the present study may attribute to DEN/2AAF-administration. Moreover, the decrease of heart GSH content, GPx, GST and SOD may due to high levels of free radicals lead to oxidative stress, which is involved in the initiation and progression of different types of cardiovascular diseases [5, 50, 51].GSH depletion and accumulation of its oxidized form, GSSG, occurred in heart muscle within minutes of initiating oxidative stress [52]. GPx, which uses glutathione as a substrate, is to reduce soluble hydrogen peroxide and alkyl peroxides [53]. Also, GPx is the most important antioxidant enzyme in humans which is highly expressed in the kidney, involved in scavenging and inactivating hydrogen and lipid peroxides, providing protection to the body against oxidative stress and also removes peroxides and peroxynitrite that can cause renal damage [54]. GST located in cytosol plays an important role in detoxification and excretion of xenobiotics [55] and catalyzes the conjugation of a variety of electrophilic substrates to the thiol group of GSH, producing less toxic forms [56]. SOD is an important scavenger of reactive oxidative species and, oxidative stress is the major cause for the development of chronic renal failure and reduction in cardiac SOD activity may due to the over production of superoxide radical onions [57].The treatment of DEN/2AAF-administered rats with avocado fruit and seed hydroethanolic extract produce a significant reduction in kidney and heart LPO level and increase in GSH content and SOD, GPx and GST activities as compared to DEN/2AAF-administered control group. These result were in agreement with Mahadeva et al. [30] and Wu et al. [58] who report that avocado fruit act as nephro-protective agent by attenuating renal oxidative stress and have the highest fruit lipophilic antioxidant capacity, which may be one factor in helping to and promoting vascular health which led to decrease LPO level and increase GSH content, GPx, GST and SOD activities. The antioxidant effects of avocado may be due to its phytochemical composition. Avocado fruit and seed contain antioxidant, phenolics and flavonoids compounds such as querectin, luteolin, rutin, kamferol and hypersoid acid [59], while flavonoid, luteolin has been shown to possess direct antioxidant activity and it is useful in the treatment of many chronic diseases associated with oxidative stress [60].The activities of these antioxidant enzymes in renal tissue were reverted back to near normal values that may due to the presence of its saponins, flavonoids and phytosterol in avocado extract [61]. Avocado could bolster the myocardial antioxidative defence system against oxidative stress which may be attributed to the free radical scavenging activities of the extract of avocado fruits [32]. The results suggest that consumption of avocado (fruit and seed) might have some cardiovascular protective properties and beneficial effects on CVD risks. So, we recommended consuming avocado seed because the avocado seed has strong antioxidant activity [62].On the other hand, several reports have demonstrated that chronic kidney disease is an important risk factor for cardiovascular disease, In fact, the adjusted hazard ratio for cardiovascular disease events increases with a decrease in the glomerular filtration rate (GFR) [63].
5. Conclusions
- The possible preventive effect of avocado fruit and seed hydroethanolic extracts on DEN/2AAF-induced cardio-nephrotoxicity may be explained on the basis of oxidant-antioxidant system management as well as regulation of the inflammatory status. Thus, these treatments may act as antioxidant and anti-inflammatory preventive agents. However, further clinical studies are required to pharmacological investigations to assess the safety and the efficacy of these extracts in human beings.
ACKNOWLEDGEMENTS
- We would like to thank Dr. Mohamed Ahmed Fadl, lecturer of taxonomy, Botany Department, Faculty of Science, Beni-Suef University, Egypt, for authenticated the Avocado pear (Persea americana) fruit.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML