-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Basic Sciences of Medicine
p-ISSN: 2167-7344 e-ISSN: 2167-7352
2017; 6(1): 1-3
doi:10.5923/j.medicine.20170601.01

Arcanobacterium Hemolyticum Chorioamnionitis Leading to Preterm Labour – A Case Report
Deepthy Balakrishnan
Department of Obstetrics and Gynaecology, Medical College, Trivandrum, India
Correspondence to: Deepthy Balakrishnan, Department of Obstetrics and Gynaecology, Medical College, Trivandrum, India.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Arcanobacterium hemolyticum has been identified as a cause of upper respiratory tract infection and pyoderma. The presence of this organism in amniotic fluid is very rare. We report a case of subclinical chorioamnionitis due to Arcanobacterium hemolyticum. Mother did not have the typical features of chorioamnionitis except for foul smelling amniotic fluid but developed preterm labour with intact membranes. The diagnosis is usually difficult as they may be viewed as contaminants or normal flora.
Keywords: Arcanobacterium hemolyticum, Preterm labour, Chorioamnionitis
Cite this paper: Deepthy Balakrishnan, Arcanobacterium Hemolyticum Chorioamnionitis Leading to Preterm Labour – A Case Report, Basic Sciences of Medicine , Vol. 6 No. 1, 2017, pp. 1-3. doi: 10.5923/j.medicine.20170601.01.
1. Introduction
- Preterm labour is known to be well associated with chorioamnionitis. The diagnostic criteria of chorioamnionitis includes clinical (presence of typical clinical findings like fever, uterine tenderness, maternal and foetal tachycardia and malodorous amniotic fluid), microbiologic (culture of microbes from appropriately collected amniotic fluid or chorioamnion) or histopathologic (microscopic evidence of infection or inflammation on examination of the placenta or chorioamnionic specimen) [1].We report a case of Arcanobacterium hemolyticum infection, an unusual organism, in foul smelling amniotic fluid of a patient who presented with intact membranes and preterm labour. Arcanobacterium hemolyticum is a pleomorphic, facultatively anaerobic, nonmotile, nonsporulating, non–acid-fast haemolytic gram-positive bacillus. [2, 3, 6] Man is the primary environmental reservoir. It has been identified as a commensal of the human pharyngeal flora and is seen in association with beta hemolytic streptococcus and other pathogens. [5, 7, 8] The organism is mainly associated with upper respiratory tract infection and pyoderma but has been occasionally isolated in patients with deep seated infections like sepsis, infective endocarditis, pyothorax and osteomyelitis. [9-11] As the organism is frequently overlooked by the Clinical Microbiology laboratory, it is important to recognise the ability of this organism to cause disease to make a correct diagnosis and commence appropriate antibiotic therapy.
2. Case Report
- 22 year old, G2P1L0infantdeath1 was admitted for evaluation of polyhydramnios. She had a 3 rd degree consanguineous marriage. Her first pregnancy was uncomplicated which went up to term [details not available] delivered a 3Kg male baby. The baby presented with abdominal distension and failure to thrive and was diagnosed to have distal tubular acidosis. He expired on 71st postnatal day due to necrotising enterocolitis. The present pregnancy was also uncomplicated except for unexplained polyhydramnios (AFI-37cm) around 34 weeks of gestation. She went in to preterm labour with rupture of membranes at 36 weeks 3days of gestation and delivered an active preterm male baby of 2.8 Kg. The amniotic fluid was copious, yellow and foul smelling with suspended inspissated material which was sent for culture. She did not have any other clinical feature of chorioamnionitis except for foul smelling amniotic fluid. She was HIV negative, not diabetic with no features of immunosuppression and her total count was 8900/mm3. Blood culture, high vaginal swab and urine culture were sterile. The amniotic fluid was sent for microscopy and for culture and sensitivity. Methylene blue stain showed gram positive bacilli with granules. Gram staining showed nonmotile gram positive bacilli and gram positive cocci in pairs and chains. Amniotic fluid culture on blood agar after 48 hours of incubation showed heavy growth of beta lytic minute colonies [small colonies with narrow zone of beta hemolysis]. Chocolate agar also showed heavy growth. Carbohydrate fermentation tests with glucose and maltose, reverse CAMP test were found to be positive. Catalase, oxidase, urease, nitrate and aesculin were negative. Mac conkeys agar showed no growth and tellurite blood agar showed no black colonies. The presence of arcanobacterium hemolyticum in amniotic fluid was confirmed which was sensitive to penicillin, erythromycin and resistant to septran. A very foul smelling placenta was sent for histopathology which showed normal histology. Her postnatal period was uneventful and the baby was admitted to NICU; found to be C- reactive protein positive and was treated with erythromycin. The baby was readmitted with abdominal distension after a week; evaluated and was diagnosed to have distal renal tubular acidosis.
 | MACROSCOPY OF AMNIOTIC FLUID |
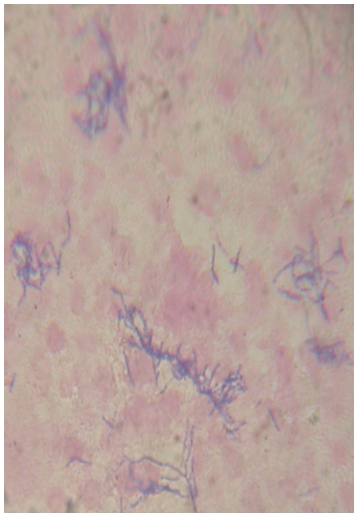 | GRAM POSITIVE BACILLUS |
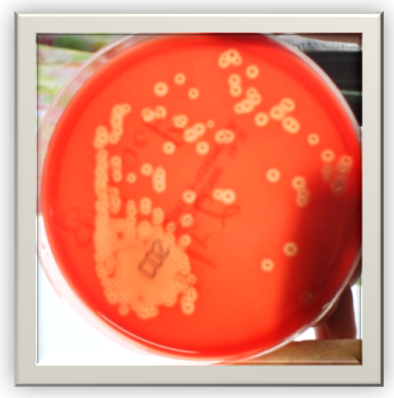 | HAEMOLYSIS ON BLOOD AGAR |
3. Discussion
- Chorioamnionitis or intraamniotic infection is an acute inflammation of the membranes and chorion of the placenta, typically due to polymicrobial bacterial infection like anaerobes and group B streptococci in the setting of membrane rupture but can occur with intact membranes by genital mycoplasmas found in genital tract of 70% of women [1, 17]. It occurs usually due to an ascending infection but rarely a hematogeneous spread can occur as in listeria monocytogenes and fusobacterium. [1, 4, 15]. Chorioamnionitis that is subclinical by definition does not present with typical clinical signs but may manifest as preterm labour or, even more commonly, as preterm premature rupture of membranes [1]. In this case, the infection was caused by a rare organism, arcanobacterium hemolyticum which was identified by gram staining, presence of colonies with hemolysis, carbohydrate fermentation tests and reverse camp test [5, 6]. Microscopic morphology differentiates Arcanobacterium hemolyticum from streptococcus species; beta-haemolysis and absence of catalase from Corynebacterium species; and lack of proteolytic activity, failure to ferment xylose, and reverse CAMP-test from A. Pyogenes [12]. The bacterial composition of amniotic fluid in ruptured membranes is usually polymicrobial. In our case, the membranes were unruptured and no other organism could be isolated. To best of our knowledge, this is the second case in which arcanobacterium has been isolated from amniotic fluid [16].The patient was asymptomatic but presented with preterm labour and premature rupture of membranes. Preterm labour could have been due to infection or polyhydramnios. The cause of polyhydramnios can be attributed to the distal tubular acidosis of the fetus as the placenta had a normal histology.Purulence or foul odour of amniotic fluid are more likely to be present with severe or prolonged infection and may be organism-specific. [1] In this case, the amniotic fluid and placenta were very foul smelling with inspissated material; the histopathologic identification could not be done. Arachnobacterium is commonly seen in individuals with HIV and diabetes but our patient is not immunocompromised. [16].
4. Conclusions
- As intrauterine infections can increase maternal and fetal mortality and morbidity, it is important to have awareness about uncommon pathogens which can cause chorioamnionitis. Arachnobacterium may be missed during routine cultures as the haemolytic pattern is not pronounced after 24 hours of incubation and hence it is important to maintain a high index of suspicion for proper management. [4] In a recent study the authors concluded that after 48 hours of incubation, trypticase soy agar with 5% horse blood in 5% was the best medium. [12]
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML