-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Basic Sciences of Medicine
p-ISSN: 2167-7344 e-ISSN: 2167-7352
2016; 5(1): 1-4
doi:10.5923/j.medicine.20160501.01

Abnormal Cord Formation of Brachial Plexus
Rajani Singh1, R. Shane Tubbs2, Raj Kumar3
1Department of Anatomy, AIIMS, Rishikesh, Rishikesh, India
2Pediatric Neurosurgery, Childern’s of Alabama, Birmingham AL, USA
3Department of Neurosurgery, AIIMS, Rishikesh, Rishikesh, India
Correspondence to: Rajani Singh, Department of Anatomy, AIIMS, Rishikesh, Rishikesh, India.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Brachial plexus is very complicated nerve network with umpteen numbers of variations. During routine dissection of a 70-year old female cadaver, an anomalous single cord was detected after fusion of all the three trunks without splitting into anterior and posterior divisions. This enhances the complexity of configuration of axons and fascicles making clinical investigation and treatment through nerve cuff electrode, functional electrical stimulation and other micro-neurosurgical interventions more cumbersome. Therefore the case is reported for a rare variant to unfold the complexity theoretically, by glimpses of fascicular anatomy. The study will be useful to anesthetists, surgeons, radiologists and anatomists.
Keywords: Brachial plexus, Variations, Single cord, Nerve cuff electrode, Fascicular anatomy
Cite this paper: Rajani Singh, R. Shane Tubbs, Raj Kumar, Abnormal Cord Formation of Brachial Plexus, Basic Sciences of Medicine , Vol. 5 No. 1, 2016, pp. 1-4. doi: 10.5923/j.medicine.20160501.01.
Article Outline
1. Introduction
- The brachial plexus is most complex and full of variations thereby least known nerve network in the entire nerve tree of the human body. Brachial plexus composed of roots, trunks, divisions, cords and nerve branches. These structures are formed by merging together and splitting up separately. Thus normally, three cords of brachial plexus are formed by fusion of anterior and posterior divisions of three trunks of brachial plexus. But in present study, three trunks without undergoing division directly fused to form anomalous single cord, a rare variant. But the nerves of brachial plexus, are composed of axons/fascicles. The messages of security from internal/external threats and alerts or commands in response to these threats or otherwise to maintain body functions, are communicated to/from the brain from/to all parts/organs of the human body. These communication channels of axons/fascicles are analogous to electrical cable [1] more explanatory, fascicles to wires and axons to strands. In this configuration of brachial plexus and its branches, one type of axonal bundles carry the sensory/ afferent information signal from sensory neurons on skin or other sensory organs through relay neurons at spinal cord communicating to brain and in response to this another type of axonal bundles transmits the command/ efferent information to various parts or organs of the body. These threats due to external/internal pathogens, toxins, drugs, environmental hazards, traumas, misuse of limbs, congenital anomalies and/or iatrogenic factors include secondary to spinal cord injury (SCI) or peripheral nerve injury cause damage/compression to nerves thereby fascicle and more specifically to axons. The damaged axons/fascicles influence the communication thereby impairment of functions of upper limb and signs and symptoms. These signs and symptoms and physical examination of the patients are used to diagnose the disease and injury. Therefore intraneural topography pertaining to fascicular anatomy gained clinical importance at fascicular level after the introduction of magnifying devices, sophisticated instruments, and microsurgical suture and their use in interfascicular dissection for the purpose of neurolysis, interfascicular grafting, and repair by surgeon [2, 3]. Because of the increasingly frequent use of these techniques, an accurate understanding of intraneural architecture has become mandatory [2]. This internal composition consisting of organization of axons/fascicles depends on variations in nerve network pattern therefore variant cord formation as observed in this study gain vital importance to comprehend fascicular arrangement crucial for diagnosis by imagery, electrical stimulation, signs/symptoms and treatment by magnifying devices, sophisticated instruments, and microsurgical suture and their use in interfascicular dissection for the purpose of neurolysis, interfascicular grafting, and repair by surgeons through nerve cuff electrode.Therefore the aim of study is to report a rare variant in the development of cord system coupled with an attempt to uncover theoretically the configuration of fascicles based on the principle of consistency, continuity and traceability in association with its impact on clinical significance [4] to facilitate anesthetists, surgeons, radiologists and anatomists.
2. Case Report
- During dissection of the upper limb on the left side of a 70- year old female cadaver, the ventral rami of C5, and C6 of spinal nerves joined to form the upper trunk, C7 continued as the middle trunk, and C8 and T1 united to form the lower trunk. These trunks anomalously fused together forming a single cord after passing for variable distances (Fig.1) but without dividing into anterior and posterior divisions in place of normal three cord configuration. This is a very rare case of abnormal configuration of cords of brachial plexus fusing in a single structure. The length of this cord was 9 mm. The case throws light on repercussion of this anomalous but rare variant and theoretically on evolution of a suitable fascicular model based on the principle of continuity, consistency and traceability of fascicles/axons on clinical maintenance of the affected upper limb. This will facilitate clinicians for diagnosis and treatment of the diseases pertaining to this zone. The single cord gave rise to all the branches normally. There was no other abnormality bilaterally.
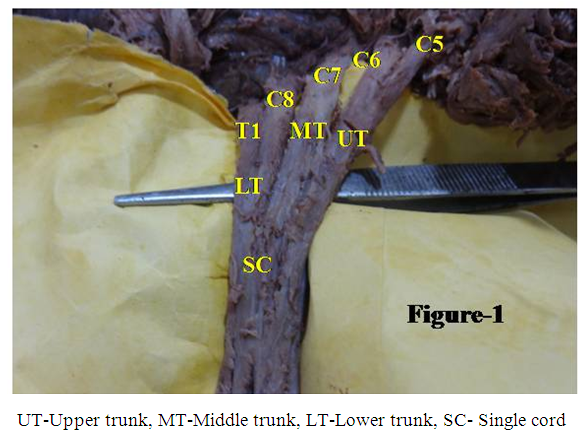 | Figure 1. Formation of a single cord by fusion of the three trunks of the brachial plexus |
3. Discussion
- Complex brachial plexus coupled with variant cord formation modifies the communication system between brain and upper limb. Therefore, to comprehend the communication system, let us compare the variations observed in cord anatomy of brachial plexus. Aggarwal et al., [5] detected formation of four single cords with three variations. Firstly the upper trunk united with the, C7, C8, and T1 roots to form a single cord. Secondly the middle and lower trunks directly fused with both the two divisions of the upper trunk, resulting in the formation of single cylindrical cord. Thirdly the upper, middle, and lower trunks fused directly to form a single cord similar to the present study. Another variant of bilateral single cord in a female cadaver of South Indian origin was reported by Viswanathan et al. [6]. In this study the C4, C5, and C6 roots on the left side combined to form the upper trunk, the C7 root continued as the middle trunk, and C8 and T1 united to form the lower trunk which instantly fused to form a single cord. The present study differs from this case in formation of the upper trunk. In Viswanathan’s [6] study on the right side in same specimen, the C5 and C6 roots joined to form the upper trunk, which divided into anterior and posterior divisions. These anterior and posterior divisions united with the lower trunk formed by fusion of the C7, C8, and T1 roots to form a single cord. In the present case the upper trunk, middle trunk and lower trunk were formed as described in standard text books. Without dividing into anterior and posterior divisions, the trunks fused to form a single cord on the left side, which is different from the case described by the above authors except third case of Aggarwal et al. [5]. The axons/fascicles are continuously reorganized and regrouped corresponding to each junction and bifurcation points where the distribution of sensory/motor axons/fascicles for targeted innervations of a particular region containing sensory and motor control points is executed. The sensory axons are distinct due to having separate origin from motor axons and so they are expected to form sensory and motor fascicles separately having different innervation zones. Here the branching of nerves may be considered requirement based distribution of innervations through fascicles /axons. Therefore number of fascicles may change because of reorganization of axons into fascicles at transformation points for bifurcation and branching but total number of axons remains constant because of fixed sensory and motor innervating control points. The analogy of nerves and their intraneural elements with electrical cables as described in introduction section not only simplifies fascicular model and their disposition to identify the fascicle, a surgical unit, for diagnosis of neuropathy in imagery and or functional electrical stimulation but also facilitates the surgeons for planning in microsurgical interventions. Though early study questions traceability of fascicles [3] but later [2] demonstrated it in distal branches. Sundarland [3] could not recognize characteristic fascicular pattern but Jabaley et al. [2] reported fibers arranged into separate bundles at distal level and formation of fascicular plexus without precluding surgical interventions. Gustafson’s [4] concept of continuity, consistency and traceability of fascicles like wires in physical communication helps in understanding the fascicular configuration branching pattern and variations. In addition to this, the analogy paves the high way to identify the damaged fascicles and site of injury to facilitate the treatment. Therefore if this theory is extended to single cord in the complex brachial plexus, the fascicular model is extremely simplified and the fascicles can be easily traced like wires in communication theory in all the nerves in this zone besides improving interpretation of advanced neurography by magnetic resonance imagery [7] and 3D ultra sonography [8, 9]. This may provide a new insight into simplification of identification, localization and traceability of fascicles/axons. This may again facilitate design, development and utilization of nerve cuff electrode treatment to neuropathy of upper extremity by surgeons.
4. Clinical Significance
- The descriptions of nerve variations in brachial plexus in general and cord formation in particular, can be the cause of a nerve palsy syndrome due to a different relation of a nerve and a related muscle [10], are useful in clinical and neurosurgical practice. The knowledge of these variations will also be useful in the surgical treatment of tumors of nerve sheaths such as schwannomas and neurofibromas [9] coupled with orthopedic procedures of the cervical spine. As the patients of neuropathy suffer from severe pain which many times is managed through anesthetic pain suppression so nerve block is performed. But certain cord variations of the brachial plexus cause an unpredictable spread of local anesthetic [11] or at times undesired blockade distribution. Possible implications of single cords include the inability to perform selective blockade of individual cords, misidentification of structures, inability to obtain a motor response, or paresthesia due to the aberrant course of the plexus [5]. The investigation of neuropathy consists of location of neural lesion, identification of nerve and fascicle. The diagnosis is done by physical examination, imagery interpretation of advanced MRI neurography/ 3D ultrasonography and lastly by FES. After this microneuro surgical interventions are planned by nerve cuff electrode. Currently available neural prostheses use muscle-based, rather than nerve-based, electrodes. Surgically implanted FES systems employing epimysial electrodes sutured to the muscle at the nerve entry point [12, 13] and/or intramuscular electrodes inserted into the muscle belly near its innervating neural structure [14] have been successful in restoring hand grasp to individuals with midcervical tetraplegia [15, 16]. The anatomical variations single cord of the brachial plexus may render it vulnerable to injury during routine surgical neck dissection [17].Therefore the knowledge of normal and variant configuration of nerves and fascicle in brachial plexus due to single cord is of paramount importance. Anomalous single cord formation provides vital clues for comprehending the configuration of nerves and fascicles to facilitate neurosurgical treatment. The complexity of brachial plexus is aggravated by the presence of variations like single cord. Therefore the medical maintenance of upper limb advances towards failure of treatment. These failures can be arrested with the development of knowledge of the variations and the fascicular anatomy of this plexus.
ACKNOWLEDGEMENTS
- There is no conflict of interest and authors have not received any financial support from any institution in relation to present article.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML