-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Basic Sciences of Medicine
p-ISSN: 2167-7344 e-ISSN: 2167-7352
2015; 4(4): 58-66
doi:10.5923/j.medicine.20150404.02

Study of Glutathione-S-Transferase, Cancer Embryonic Antigen and Carbohydrate Antigen 19-9 in Ulcerative Colitis and Colorectal Cancer
Fatma Saffeyeldin Mohamed1, Mirhan M. Elkady2, Eman Fekry Mohamed3, Boshra E. Hussein4, Walid S. H. Elsaied5
1Department of Tropical Medicine, Faculty of Medicine (for girls), Al-Azhar University, Cairo, Egypt
2Department of Clinical Pathology, Faculty of Medicine (for girls), Al-Azhar University, Cairo, Egypt
3Department of Internal Medicine, Faculty of Medicine (for girls), Al-Azhar University, Cairo, Egypt
4Department of Tropical Medicine, Faculty of Medicine, Tanta University, Tanta, Egypt
5Department of Pathology, Faculty of Medicine, Zagazig University, Zagazig, Egypt
Correspondence to: Fatma Saffeyeldin Mohamed, Department of Tropical Medicine, Faculty of Medicine (for girls), Al-Azhar University, Cairo, Egypt.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Background: Ulcerative colitis (UC) is a chronic inflammatory bowel disease (IBD) with a high risk of colorectal cancer (CRC), which is a major cause of cancer morbidity and mortality worldwide. Glutathione-S-transferase (GST) has an essential role within the cells, including the conjugation and detoxification of toxic or carcinogenic compounds, such as reactive oxygen species (ROS). Cancer embryonic antigen (CEA) and carbohydrate cancer antigen (CA19-9) are tumor markers that are used for diagnosis of colorectal cancer. Aim: The aim of the study was to assess the total plasma GST activity, CEA and CA 19-9 in ulcerative colitis and colorectal cancer. Subjects and methods: The plasma GST activity, CEA and CA 19-9 were assessed in 40 patients with CRC (group I), 40 patients with UC (group II) and 20 apparently healthy individuals as controls (group III). Results: The mean GST activity, CEA and CA19-9 were significantly higher in CRC and UC patients than controls with no significant difference between CRC & UC patients in CEA or CA19-9. A significant difference was found between Gs I & II as regards GST activity. Conclusion: Measurement of the total plasma GST activity may be helpful as a tumor marker of colorectal cancer.
Keywords: Glutathione-S-Transferase (GST), Colorectal cancer (CRC), Ulcerative colitis (UC), CEA & CA19-9
Cite this paper: Fatma Saffeyeldin Mohamed, Mirhan M. Elkady, Eman Fekry Mohamed, Boshra E. Hussein, Walid S. H. Elsaied, Study of Glutathione-S-Transferase, Cancer Embryonic Antigen and Carbohydrate Antigen 19-9 in Ulcerative Colitis and Colorectal Cancer, Basic Sciences of Medicine , Vol. 4 No. 4, 2015, pp. 58-66. doi: 10.5923/j.medicine.20150404.02.
Article Outline
1. Introduction
- Inflammatory bowel disease is a chronic inflammatory condition affecting the gastrointestinal tract. It has two main forms, ulcerative colitis (UC) and Crohn’s disease [1]. Ulcerative colitis affects only the large bowel, and the inflammatory process is confined to the mucosa. Ulcerative colitis is a well-known risk factor for colorectal cancer [2]. The possibility of UC developing to intestinal cancer has a close and positive relationship with the extent and duration of the disease [1].Colorectal cancer (CRC) is a major health concern and a leading cause of cancer death in the western world. The estimated life time risk of CRC is 5% to 6%. Worldwide, it is estimated that there was over one million new CRC cases and the annual age-adjusted incidence rate is 57 per 100000 [3]. Survival is directly related to the extent of disease at the time of diagnosis. Those diagnosed at an advanced stage have an estimated 5-year survival rate of 7%, in contrast with a survival rate of 92% for individuals detected at an early stage, since advanced CRC is largely refractory to conventional therapy and is one of the least curable malignancies [4].Despite continuing advances in diagnosis and therapy, long-term survival has not improved significantly over the last four decades, and almost 50% of CRC patients will eventually die of their disease [5]. This situation mandates improvement in the early detection of this surgically curable disease and preventative interventions to reduce the incidence of the disease and its morbidities and mortalities [6]. Indeed, given our understanding of the pathogenesis of CRC, current screening technologies, and effective preventative interventions, CRC should be highly preventable [7].The colon and rectum are constantly challenged by potentially harmful compounds, including mutagens and carcinogens [7]. The large intestine possesses several defense mechanisms to counteract damage of the colorectal mucosa by such reactive compounds [8]. These include the ability to up-regulate detoxification systems, essentially, the glutathione (GSH) / glutathione-S-transferase (GST) detoxification system [9].Glutathione-S-transferases (GSTs) are enzymes with detoxifying properties that are ubiquitously expressed, with especially high levels in the liver, gonads and colon [1].These enzymes are expressed in a wide variety of human tissues, including both normal and malignant colonic mucosa [10]. Most human gastrointestinal tumors contain increased GST enzyme activity which has been suggested as being beneficial to cancer prevention [11]. Previous studies have shown some evidence for a positive association between GST gene mutations and the susceptibility to multiple tumors [1].The most important currently available markers in CRC that provide prognostic or predictive information are serum markers such as CEA and CA19-9, expressed by tumor tissue [12]. Carcinoma embryonic antigen (CEA) is an oncofetal tumor marker in 70% of cases. It is significant in the diagnosis of colorectal cancer. Increase of its concentration for a period of a few months after the surgery help in prediction and diagnosis of recurrence [13]. Its concentration is also correlated with the tumor size. Thus, tumors of smaller size have normal serum concentrations of CEA antigen. Only tumors greater than 3 cm are accompanied with high concentration of CEA antigen [14]. Carbohydrate antigen (CA19-9) is a cancer antigen whose elevated serum concentration is also detected in the case of colorectal cancer. It is a tumor marker that is observed to be in elevated serum concentration with metastatic colon cancer [12, 15].
2. Subjects and Methods
- The study was conducted on 40 patients with CRC (group I), 40 with UC (group II) and 20 apparently healthy controls (group III). Mean ages of participants were 50.05±11.43, 49.25±9.42 and 48.25±15.68 in groups I, II & III, respectively.Patients included in this study were selected from outpatient clinics and inpatients of Internal and Tropical Medicine Departments, Alzahraa University Hospital, Cairo, Egypt. Group (I) included 27 males and 13 females with CRC, group (II) included 18 males and 22 females with UC and group (III) included 10 males and 10 females as controls. Patients with liver disease or high bilirubin were excluded as both affect GST enzymatic activity, thus decreasing the clinical sensitivity of the test [16]. A verbal consent was taken from all patients and controls.All participants were subjected to: thorough history taking, full clinical examination and the following routine laboratory investigations; Liver function tests (Alanine transaminase ALT, aspartate transaminase AST, alkaline phosphatase ALP, total and direct bilirubin, albumin and total proteins), kidney function tests (Urea and Creatinine).Estimation the total plasma GST enzyme activity, CEA and CA19-9 were assayed in serum samples using enzyme –linked immunosorbent assay (ELISA) in all patients and controls. Abdomino-pelvic ultrasound was also done. CT was done for patients with CRC. Colonoscopy, biopsy and histopathological examination were done for all patients.
2.1. Measuring the total GST Activity in Plasma
- Plasma total GST activity was measured in all patients and controls using enzyme–linked immunosorbent assay (ELISA). The Cayman Chemical Glutathione S-transferase assay Kit (Catalog No. 703302) was supplied from Cayman Chemical company, USA. Plasma total GST activity kit measures the conjugation of 1-chloro-2, 4-dinitrobenzene (CDNB) with reduced glutathione. The conjugation is accompanied by an increase in absorbance at 340nm. The rate of increase is directly proportional to the GST activity in the sample. The Cayman GST Assay Kit can be used to measure GST activity in plasma.
2.2. Quantitative Measurement of CEA
- The CEA enzyme immunoassay test kits (Catalogue No. EK-310-11) were supplied from Phoenix Pharmaceuticals, Inc., CA, USA. The CEA ELISA test is based on the principle of a solid phase enzyme linked immunosorbent assay. The assay system utilizes a monoclonal antibody directed against a distinct antigenic determinant on the intact CEA molecule is used for solid phase immobilization (on the microtiter wells). A goat anti-CEA antibody conjugated to horseradish peroxidase (HRP) is in the antibody-enzyme conjugate solution. The test sample is allowed to react simultaneously with the two antibodies, resulting in the CEA molecules being sandwiched between the solid phase and enzyme-linked antibodies. After a 1 hour incubation at room temperature, the wells are washed with water to remove unbound labeled antibodies. A solution of TMB Reagent is added and incubated for 20 minutes, resulting in the development of a blue color. The color development is stopped with the addition of stop solution changing the color to yellow. Absorbance is measured at 450 nm.
2.3. Quantitative Measurement of CA19-9
- The CA19-9 enzyme immunoassay test kits (Catalogue No. TM E-4500) were supplied from LDN Labor Diagnostika Nord GmbH Co. KG, Germany. The TM-CA19.9 ELISA Kit is a solid phase enzyme-linked immunosorbent assay (ELISA) based on the sandwich principle. The microtiter wells are coated with a monoclonal antibody directed towards a unique antigenic site of the CA 19-9 molecule. An aliquot of patient sample containing endogenous CA 19-9 is incubated in the coated well with assay buffer. After a washing step a second incubation follows with enzyme conjugate, which is an anti-CA 19-9 antibody conjugated with horseradish peroxidase. After incubation the unbound conjugate is washed off. The amount of bound peroxidase is proportional to the concentration of CA 19-9 in the sample. Having added the substrate solution, the intensity of color developed is proportional to the concentration of CA 19-9 in the patient sample.
2.4. Statistical Methods
- The collected data were coded, fed to a computer, organized and statistically analyzed. Statistical analyses were used computer programs: Microsoft excel version 10 and Statistical Package for Social Science (SPSS) for windows version 20.0 [17].A) Descriptive statistics:1. Qualitative (categorical) data were presented by frequency and percentage.2. Quantitative data were presented by mean ± SD. B) Analytical statistics: Comparing groups was done using1. Student “t "- test: was used for comparison between mean of two groups as regard quantitative variables.2. Analysis of variance [ANOVA (F)] test: was used for comparison of quantitative data of more than 2 groups. C) Mann-whitney (Z test) and Kruskal Wallis Test were used for non-parametric data. D) Pearson linear correlation coefficient (r) was estimated to show the relationship between quantitative parameters [17].E) Receiver operating characteristic (ROC) curves to determine a cutoff value. The optimal value of the cut-off point is thus obtained when the sum of sensitivity and specificity is at its maximum [18].
3. Results
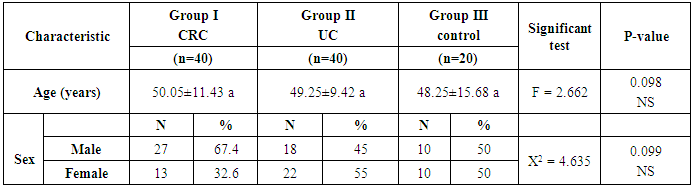
- Patients with CRC (group I) included 40 patients [27 males (67.4%) and 13 females (32.6%)], group II (UC) included 40 patients [18 males (45%) and 22 females (55%)] and group III included 20 apparently healthy controls [10 males (50%) and 10 females (50%)]. Mean ages of participants were 50.05±11.43, 49.25±9.42 and 48.25±15.68 in groups I, II & III, respectively (Table 1 and Figures 1 & 2).
|
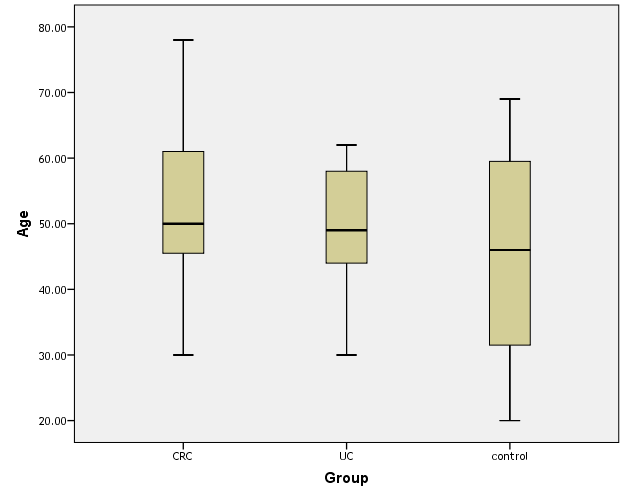 | Figure (1). Box plot of groups as regards age and their statistical difference |
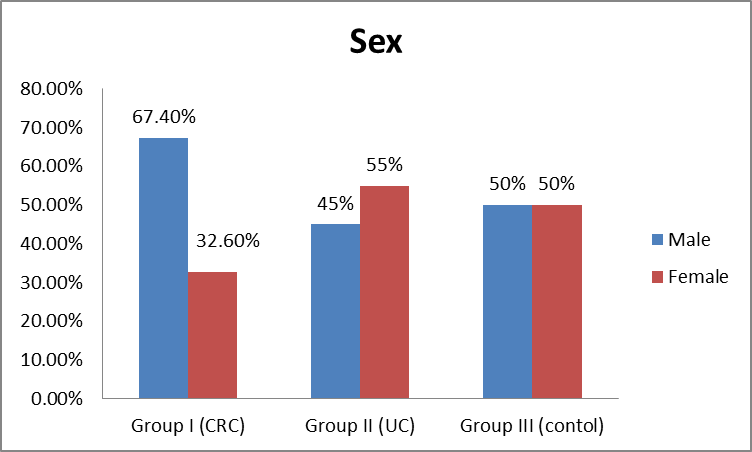 | Figure (2). Distribution of sex among the studied groups |
|
|
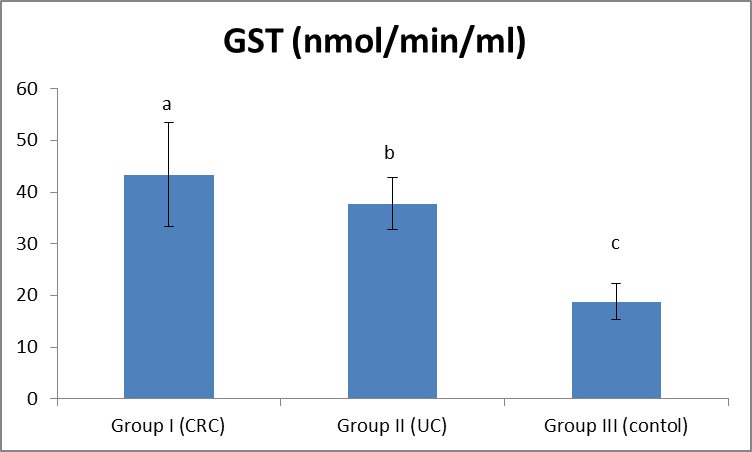 | Figure (3). Comparison between both groups as GST and their statistical significance |
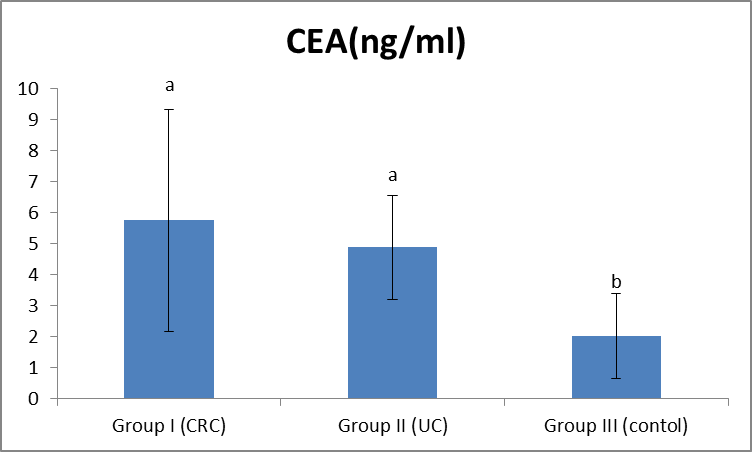 | Figure (4). Comparison between both groups as CEA and their statistical significance |
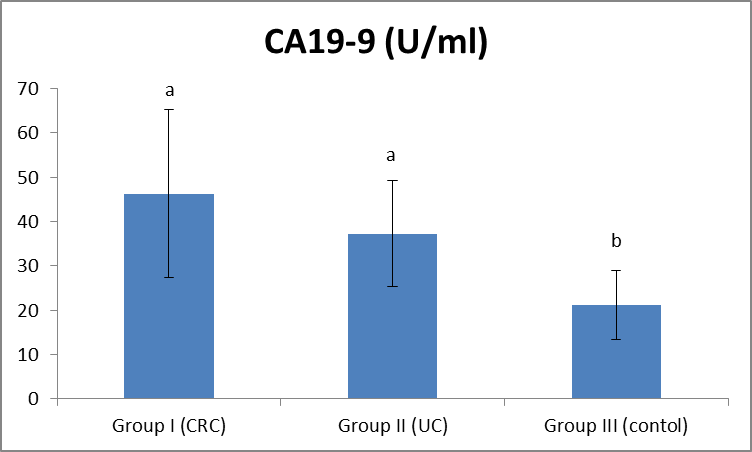 | Figure (5). Comparison between both groups as CA19-9 and their statistical significance |
|
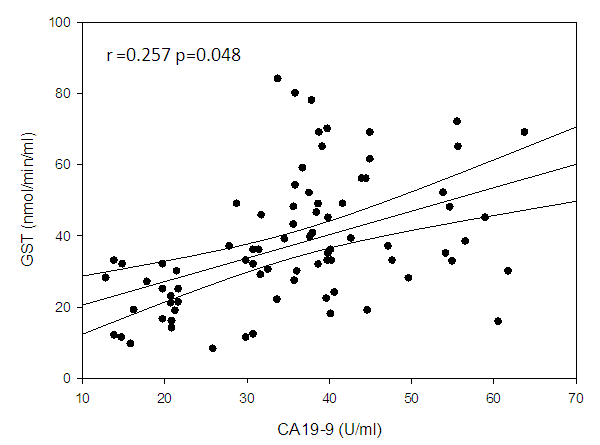 | Figure (6). Linear correlation between GST and CA19-9 and their statistical significance |
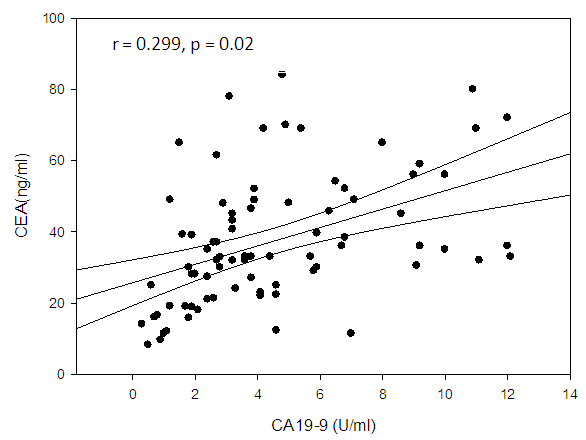 | Figure (7). Linear correlation between CEA and CA19-9 and their statistical significance |
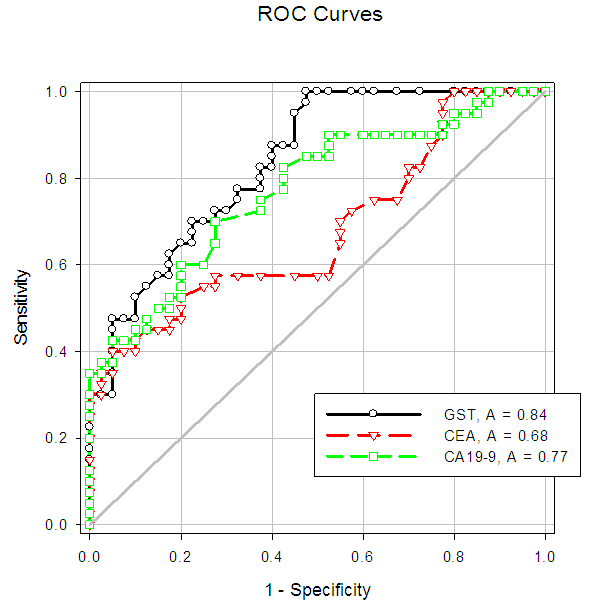 | Figure (8). Sensitivity and specificity of CEA and plasma GST activity in the CRC patients shown in the ROC curve |
4. Discussion
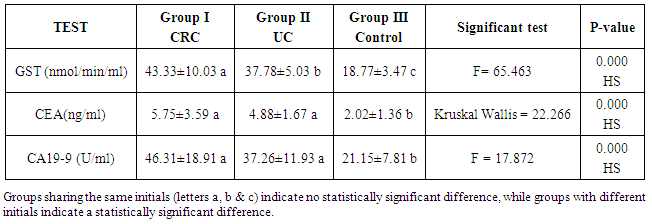
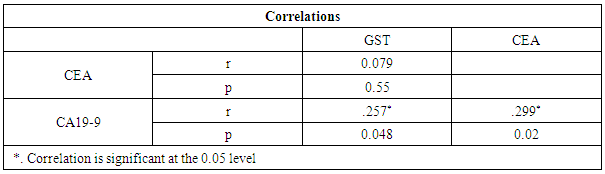
- Colorectal cancer (CRC) is a major health concern and a leading cause of cancer death in the western world [3]. Ulcerative colitis is a well known risk factor for colorectal cancer [2].The most important currently available markers in CRC that provide prognostic or predictive information are serum markers such as CEA and CA19-9, expressed by tumor tissue [12]. Previous studies have shown some evidence for a positive association between GST gene mutations and the susceptibility to multiple tumors [1].The present study had demonstrated a significant elevation in the plasma GST activity in CRC patients compared to UC and the control group. This was in agreement with Naidu et al. [19] and Nomani et al. [20], they compared GST activity levels in paired samples of colorectal cancer, adenoma and adjacent normal mucosa.The results of the present study were also in agreement with Severini [21], who found that the mean value of the GST activity was significantly elevated in patients with CRC as compared with the reference population. He suggested that GST measurement may be useful as a tumor marker in CRC. Moreover, the combined determination of GST and other markers increase the sensitivity for cancer detection.Butler et al. [22] measured the glutathione (GSH) concentrations and GST activity in adenomatous polyps, cancer and normal mucosa. They found that concentrations of GSH were significantly higher in adenomas and cancer than in uninvolved mucosa while GST activity was significantly higher in cancer and these results wee in agreement with our results.In contrary to the results of the present study, Upadhya et al. [23] and Ozdemirler et al. [24] demonstrated that plasma GST did not show any significant change between CRC patients and controls.A significant elevation was also found in plasma GST activity in cases with UC, compared to the control group, this was in agreement with Nieto et al. [25], they perfomed the study on experimental rats. However, our results were not in agreement with Bhaskar et al. [26], they found a significant decrease in the activity of GST activity in patients with UC compared to controls. They speculated that the reduced activity of GST in UC may be an additional factor in the pathogenesis of mucosal damage in this disease.In the present study there was a significant increase in serum CEA in CRC and UC patients than controls, with no significant difference between CRC and UC patients. This was in agreement with Vukobrat-Bijedic et al. [27], they found a significantly elevated serum concentration of CEA in cases of colon cancer with already developed metastases.The results of the present study, as regards CEA, were also in agreement with Carpelan-Holmstrom et al. [28], they evaluated and compared serum tumor markers, CEA & CA19-9, and their value in the diagnosis of malignant colorectal disease and concluded that only CEA provided a significant diagnostic information. In their a study ROC curve was constructed, and the area under the curve (AUC) was determined for the probability of cancer. Of the individual markers, the highest AUC was observed for CEA (AUC = 0.746). In the present study, According to the ROC curve, the highest Area Under the Curve (AUC) was for GST (AUC = 0.846).Also, Zhao et al. [29] concluded that CEA levels in CRC patients were significantly higher than those in controls and that was in agreement with our results. In the present study, a significant increase was found in CA19-9 in CRC and UC than controls, with no significant difference between the first two groups (Gs I & II). This was in agreement with Basbug et al. [30], they found that the mean values of CA19-9 were elevated in CRC than controls. They concluded that CA19-9 should be used for the study of this kind of malignancy. However, Carpelan-Holmstrom et al. [28], Cerda et al. [31] and Morita et al. [32] found no significant difference in CA 19-9 values between cancer and control groups and this was not in agreement with our results. Also Morita et al. [32] did not recommend routine use of CA19-9 in the staging and surveillance of colorectal cancer patients.
5. Conclusions
- GST activity, CEA and CA19-9 were significantly elevated in CRC and UC with no significant difference between both groups in CEA or CA19-9. While, GST activity showed a statistically significant increase in CRC than UC patients.
6. Recommendations
- Further studies for evaluation of GST in UC and its usefulness as a marker for CRC.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML