-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Basic Sciences of Medicine
p-ISSN: 2167-7344 e-ISSN: 2167-7352
2015; 4(3): 38-51
doi:10.5923/j.medicine.20150403.02
Effect of Sitagliptin (Januvia) on the Thyroid Gland of Adult Male Albino Rats in an Experimental Model of Type II Diabetes Mellitus - (Biochemical, Histological and Immunohistochemical Studies)
Olfat A. Abd-El Aty1, Sahar Badr El-Din2
1Departments of Anatomy, Faculty of Medicine Al-Azhar – University (Girls)
2Departments of Pharmacology, Faculty of Medicine Al-Azhar – University (Girls)
Correspondence to: Olfat A. Abd-El Aty, Departments of Anatomy, Faculty of Medicine Al-Azhar – University (Girls).
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
Type II diabetes mellitus is a major global health problem and there is ongoing research for new treatments to manage the disease. The glucagon-like peptide-1 receptor (GLP-1R) controls the physiological response to the incretin peptide, GLP- 1, Sitglaptin, is a DPP- IV inhibitor and through its effect on receptor GLP-1 seems to have much wider effects on the function and survival of cells that express its receptor. Also, inhibitors of dipeptidyl peptidase-IV (DPP-IV) are a novel class of anti-diabetes drugs; inhibiting the breakdown of incretins, they increase their biological availability and thus decrease blood glucose level. In addition to regulating glucose homeostasis, DPP-IV has many diverse functions, such as modulating cell growth, cellular differentiation and transformation and it can disturb the immune function. Effect of DPP-IV inhibitors in humans are scarce, it has a risk of infections and the tendency towards a higher incidence of some tumors which fall in line with experimental evidence suggesting the possibility of their adverse immunological and oncological effects. Aimofwork: This study was set to evaluate the possible side effects of Sitagliptin on the thyroid gland of the adult male rats after induction of diabetes mellitus II by Alloxan. MaterialandMethods: Thirty adult male albino rats were divided into three equal groups: control group, diabetic non treated group and diabetic rats treated with therapeutic dose of Sitagliptin (100 mg/kg/day) for eight weeks. At the end of the experimental period, the hormones of thyroid function, calcitonin in addition to histological, ultra structural and immune-cytochemical examinations of the thyroid tissue calcitonin and caspase 3. Also, evaluation of the oxidative markers were carried. Theresults:showed that treatment with Sitagliptin caused significant increase in free T3 and calcitonin level (P<0.001) with significant increase of oxidative markers (P<0.001). Histological examination showed evidence of stratification and focal hyperplasia of the follicular cells with strong PAS reaction in addition to engorgement of Golgi system and dilatation of the rough endoplasmic reticulum with presence of multiple secondary lysosomes. Immunohistochemical results showed significant increase (P<0.001) of calcitonin immunopositive expression of thyroid C-cells which appeared stronger than normal, in addition to marked significant increase (P<0.001) in area percent of caspase-3 immunopositive cells which appeared as massive dense brown cytoplasmic deposits.Conclusions:Sitagliptin administration induced changes in the thyroid tissues leading to quantitative and qualitative alterations in the hormonal levels and enhancement of apoptosis.
Keywords: Sitagliptin, GLP-1 receptor, DPP-4 inhibitor, Thyroid gland, Histopathology, Immunohistochemical, calcitonin, Caspase 3
Cite this paper: Olfat A. Abd-El Aty, Sahar Badr El-Din, Effect of Sitagliptin (Januvia) on the Thyroid Gland of Adult Male Albino Rats in an Experimental Model of Type II Diabetes Mellitus - (Biochemical, Histological and Immunohistochemical Studies), Basic Sciences of Medicine , Vol. 4 No. 3, 2015, pp. 38-51. doi: 10.5923/j.medicine.20150403.02.
1. Introduction
- Type II diabetes mellitus is a global public health problem, with worldwide prevalence increasing exponentially and future projections estimating that almost 10% of the adult population will suffer from that condition by the year 2030 (Shaw et al., 2010). Despite the number of anti-diabetes medications currently available, there is still difficulty achieving tight glycemic control in patients with type II diabetes (Leong et al., 2013). An emerging class of anti-diabetes agents, known as incretin-based therapies, like Sitagliptin, enhances or replaces the glucose dependent glucoregulatory effects of incretin hormones, primarily glucagon-like peptide-1 (GLP-1) (Campbell and Drucker, 2013). GLP-1 is an incretin hormone released after meals by L cells in the ileum. Native GLP-1 regulates the postprandial rise in blood glucose by augmenting insulin release and blunting glucagon secretion, delaying gastric emptying and improving satiety. These effects are short lived, as the active hormone is rapidly degraded by the enzyme dipeptidyl peptidase-4 (DPP-4) (Woerle et al., 2012). There are two types of incretin-based therapies, the GLP-1 receptor agonists and DPP-4 inhibitors. DPP-4 inhibitors have been developed and have been shown to improve fasting and postprandial glucose control with minimal hypoglycemia. They also, induce weight loss to varying extents based on their relative stimulation of incretin activity (Tibaldi, 2014).Sitglaptin, is a DPP-4 inhibitor and through its effect on receptor GLP-1 seems to have much wider effects on the function and survival of cells that express its receptor. In vitro, insulin-gene transcription is stimulated while cell apoptosis is inhibited by GLP1 receptor activation and it also stimulates cell growth. Activation of the GLP1 receptor pathway might have positive (i.e. restoration of the functional β cell mass) or negative (i.e. inducing proliferation of premalignant lesions) effects when chronically used for the treatment of type 2 diabetes (Stoffers et al., 2000).GLP-1 receptors (GLP-1R) are widely expressed in pancreatic islet cells and in several other tissues including heart, lung, vascular smooth muscle cells, endothelial cells, macrophages, monocytes, kidney, thyroid C-cells, gastrointestinal tract (stomach, liver and intestine), skeletal muscle, adipose tissue as well as in central and in peripheral nervous system and pituitary gland (Martin et al., 2011 and Gier et al., 2012).Effects on thyroid function have attracted lots of attention because thyroid hormones act on cells of almost all tissues and therefore are involved in several physiological processes during life span (Sun et al., 2008). In adults, thyroid hormones are involved in metabolism of protein, lipid and carbohydrate and in heat generation (Kim, 2008). They are also necessary for normal reproductive functions, regulation of heart rate and gastrointestinal motility, as well as for emotional stability (Bauer et al., 2008). Disruptions of thyroid function by endogenous or exogenous factors may produce various subclinical effects (Surks et al., 2004 & Havre et al., 2008) or direct clinical manifestations (Dallaire et al., 2009 & Matteucci and Giampietro, 2009).There is a deep underlying relation between diabetes mellitus and thyroid dysfunction (Brenta et al., 2007). The prevalence of thyroid disorder in diabetic population was reported to be 13.4% with higher prevalence (31.4%) in female patients as compared to (6.9%) in male patients considerably, diabetes mellitus type II patients were more prone to thyroid disorders (Duntas et al., 2011).Calcitonin, a hormone secreted by thyroid C-cells, is regarded as an important clinical biomarker for C-cell diseases such as medullary thyroid carcinoma and hereditary C-cell hyperplasia because of its high sensitivity and specificity (Elisei et al., 2004; Costante et al., 2007 and Machens et al., 2009). In humans, it has recently been reported that the Sitagliptin was consistently expressed in C-cells in neoplastic and hyperplastic lesions and in about one-third of normal C-cells in thyroids using immunofluorescence and immunohistochemistry (Gier et al., 2012).Apoptosis is a particular type of programmed cell death that is characterized by the expression of pro-apoptotic genes and the activation of a family of cystein-proteases called caspases (Taylor et al., 2008). Among them, caspase-3 which is a key executioner of apoptosis, which is activated by an initiator caspase such as caspase-9. The activated caspase-3 could cleave the poly (ADP-ribose) polymerase which is one protein related to a number of cellular processes involving mainly DNA repair and programmed cell death (Herceg and Wang, 2001). Apoptosis can occur through several different initiating processes including growth factor deprivation, toxic actions of radiation or chemotherapy drugs and through endogenous molecules that act as receptors for cell-bound or soluble ‘death’ ligands. These ligands include tumor necrosis factor (TNF), TNF-related apoptosis-inducing ligand (TRAIL) and Fas ligand (FasL). When the activating events start different signal cascades will feed into a final common apoptosis pathway that leads to cell death (Strasser et al., 2000).Few researchers are concerned with the evaluation of the structure and function of C cells in the thyroid gland or the probable relationship between these cells and the follicular cells in physiological and pathological conditions. For this reason, the current study designed to illustrate the effect of the therapeutic dose of Sitagliptin on the thyrocytes and C cells of the thyroid gland by hormonal, chemical, histological, ultra structural and immunohistochemical investigations which carried out in rats of experimental model of diabetes mellitus type II.
2. Material and Methods
- A) Experiment:30 adult male albino rats (weight 200-220gm) were housed at the animal house in the faculty of medicine for girls Al-Azhar –University at 21°C–22°C in a 12 hr/12 hr light/dark cycle, fed standard rat chow, and given free access of water. Rats acclimatized to the surroundings for 2 weeks prior to the experiments. The rats were then divided into three equal groups, of 10 rats/each as following:Group I: Control rats without receiving anything.Group II: Rats model of diabetes mellitus type II not receive any treatment.Group III: Rats model of diabetes mellitus type II received therapeutic dose of Sitagliptin (10 mg/kg/day orally for 8 weeks) according to Bipin et al. (2013).Experimental protocols of induction of diabetes mellitus type II:In humans, diabetes mellitus is one of the most prevalent conditions with spontaneous manifestation. In animals, it can be induced by partial pancreatectomy or by the administration of diabetogenic drugs such as Alloxan, Streptozotocin, Ditizona and Anti-insulin serum. These agents selectively destroy the Langerhans islet ß-cells. The best known drug-induced diabetes model is Alloxan. Alloxan, a derivative of uric acid, as well as of other substances of different chemical groups, cases ß-cells to degranulate and consequently degenerate. Alloxan induces irreversible diabetes mellitus after 24 hours following its administration and the condition proves to be chronic by laboratory tests after seven days (Macedo et al., 2002 and Ferreira and Pinto, 2010).The rats of group II and III were fasted overnight for at least 8 hours. Hyperglycemia was induced in each fasted rat by administering alloxan monohydrate (150 mg/ Kg body weight; intraperitoneal) in normal saline. At 7 days post-induction of hyperglycemia, blood glucose was assayed by the glucose oxidase method, using a glucometer. Only those rats with established hyperglycemia (blood glucose > 200 mg/dl) were included for subsequent treatment (Oluwole et al., 2012).B) Chemicals:Sitagliptin (Januvia® tablet 100mg) was obtained from Merck Sharp & Dohme Ltd. Hertfordshire EN11 9BU.United Kingdom/Royaume-Uni.Alloxan (Sigma Chemicals Company, USA): It is a 5, 6 dioxyuracil monohydrate. It is a white powder dissolved in citrate buffer (pH: 4.5) by the use of pH-meter (Genway) its molecular weight is 160.1 (Lenzen, 2008).At the end of the experiment (8 weeks) the animals were anesthetized by ether to avoid the effect of stress of manipulation on hormonal levels; blood was collected through the retro-ocular puncture. Thereafter, blood was centrifuged (3000 rpm for 20 min), and sera were separated and stored at -20 Co. Then all studied animals were sacrificed, the thyroid glands were excised one lobe prepared to biochemical study and the other lobe divided into pieces for histopathological studies by light and electron microscopies.C) Hormonal Assay:1- RIA for serum TSH and Thyroglobulin:Serum levels were determined with Amerlex RIA kits (Amersham International, Buckinghamshire, UK), according to the manufacturer’s instructions.2-Measurement of free T3:Free T3 was measured with a solid phase 125I RIA from Diagnostic Products. The euthyroid range of free T3 is 2.2–6.8 pmol/liter. This assay cross - reacts only 0.00008% with T4. The T3 analog tracer does not bind to either TBG or albumin. The intra- and inter assay coefficients of variation for the free T3 assay were 5% and 6.9%, respectively.3- Measurement of free T4:Free T4 was measured with a solid phase 125I RIA from Diagnostic Products. The euthyroid range of free T4 is 10.3–25.7 pmol/liter, and this assay’s detection limit is 1.3pmol/liter. The intra- and inter assay coefficients of variation for free T4 assay were 5% and 8%, respectively. Importantly, the analog tracer in this free T4 assay does not bind toT4-binding globulin (TBG).4. Measurement of Calcitonin (CT):CT was measured by a radioimmunoassay (Calcitonin Kit, Mitsubishi Chemical Medicine Corporation, Tokyo, Japan) that had a lower detection limit of 12.5 pg/mL and an intra-assay coefficient of variation was <10%.D) Biochemical oxidative parameters: The thyroid lobe is excised, rinsed in ice-cold 0.175 M KCl / 25 mM Tris–HCl (pH 7.4) to remove the blood, minced in the same solution, and homogenized by means of a homogenizer with a Teflon pestle. The thyroid homogenates were centrifuged at 10,000 rpm for 15 min. The supernatants were then used for lipid peroxidation determination, and antioxidant enzyme assays as follows:a) Tissue Glutathione (GSH) Analysis: The reduced GSH content of thyroid tissue was estimated according to the method described by Sedlak and Lindsay (1968). b) Tissue superoxide dismutase (SOD) and catalase (CAT) activity determination: The SOD activity was measured by the inhibition of nitrobluetetrazolium (NBT) reduction due to O2 generated by the xanthine/xanthine oxidase system (Sun et al., 1988). One unit of SOD activity was defined as the amount of protein causing 50% inhibition of the NBT reduction rate. The CAT activity of tissue was determined according to the method of Sinha (1991). The enzymatic decomposition of H2O2 was followed directly by the decrease in absorbance at 240 nm. The difference in absorbance per unit time was used as a measure of CAT activity. The enzyme activity was given in U/mg of protein.c) Determination of malondialdehyde levels (MDA): The levels of MDA in homogenized tissue, as an index of lipid peroxidation, were determined by a thiobarbituric acid reaction using the method of Yagi (1998).d) Determination of protein content: The tissue protein content was measured according to Cannon (1974) using bovine serum albumin as a standard.E) The histological Preparation for light microscopical study:The thyroid lobes were fixed in Bouin’s solution for 48 hs. Later, they were dehydrated in graded levels of ethanol, cleared in xylene, and embedded in paraffin wax for sectioning. The 4-μm thick sections were cut, mounted on glass slides, and stained with hematoxylin and eosin stain, periodic acid-Schiff (PAS) reactions, Masson’s trichrome stain and toludine blue stain for light microscopic analysis (Bancroft and Gamble, 2008).Immunohistochemical staining for detection of calcitonin:An immunohistochemical reaction used for detecting calcitonin in C cells was conducted on 4 µm-thick paraffin thyroid sections. In this procedure specific rabbit antisera against calcitonin, which can be found only in C cells, were used. The ABC (avidin-biotin peroxidase complex) method was applied according to Hsu et al. (1981).Immunohistochemical staining for detection of caspase 3:4 µm-thick paraffin thyroid sections were incubated overnight with the primary rabbit polyclonal anti-caspase-3 antibody (AbcamInc). Caspase-3 immunoexpression was detected in the cytoplasm of epithelial cells lining the follicles and was used as a marker of apoptosis. Tissue sections were counterstained with Mayer’s hematoxylin (Sanii et al., 2012).F) The histological Preparation for Transmission Electron Microscopy (TEM): Small pieces of the thyroid tissue were immediately placed in 5% gluteraldehyde buffered at pH 7.4 with Milloning phosphate for 4 hours and subsequently fixed in 1% osmium tetraoxide for two hours. The samples were dehydrated in graded ethanol and embedded in araldite. Thin sections were stained with lead citrate and uranyl acetate according to (Bancroft and Gamble, 2008) and examined with a JEOL 1010 Transmission Electron Microscope at the Regional Center for Mycology and Biotechnology (RCMB), Al-Azhar University Cairo. G) Morphometric analysis:Quantitative morphometric measurements using ‘Leica Quin 500’ software image analyzer computer system (Leica image system Ltd, Cambridge, England). The measurement were done using area percent which assessed in each parameter by reading 10 non overlapping fields for each animal at a magnification of × 400, as following :1- Area percent of colloid in PAS reaction.2- Area percent of metachromasia of mast cell in Toludine blue stain.3- Area percent of positive calcitonin immunostaining.4- Area percent of positive caspase 3 immunostaining.H) Statistical analyses:One-way analysis of variance (ANOVA) followed by LSD tests were used to compare the means among the studied groups. The data obtained in the present study were expressed as mean ± SD for quantitative variables and statistically analyzed by using SPSS program (version 17 for windows) (SPSS Inc. Chicago, IL, USA). P value <0.05 was considered statistically significant.
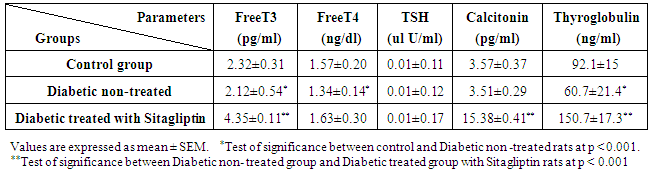
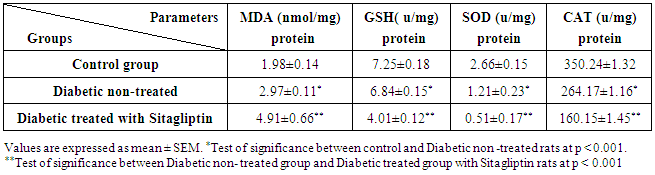
3. Results
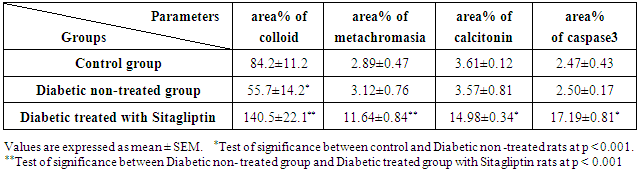
- Biochemical Results:The current study indicated that the freeT3 (pg/ml), T4 (ng/dl) and TSH (ul U/ml) serum levels in control group was 2.32±0.31, 1.57±0.20 and 0.01±0.11 (Mean ± SEM) respectively. The free T3, T4 and TSH serum levels in Sitagliptin treated group were 4.35±0.11, 1.63±0.30 and 0.01±0.17 respectively. There were significant increase in free T3 serum level in the Sitagliptin treated group when compared with the control and diabetic non-treated groups (p<0.001). As regards, the calcitonin serum levels (pg/ml) in control group were 3.57 ± 0.37. In Sitagliptin treated group the calcitonin serum levels were 15.38±0.41. There were significant increases in calcitonin serum levels in the Sitagliptin treated group when compared with the control and diabetic non-treated groups (p<0.001). In Sitagliptin treated group, the thyroglobulin serum levels were found to be significantly increase than those in control and diabetic non-treated groups (p<0.001).Biochemical oxidative parameters: The thyroid contents of the GSH, SOD and CAT activities were significantly decreased in Sitagliptin treated rats compared to those in control and diabetic non-treated rats (p<0.001). As regards the thyroid MDA content, Sitagliptin treated rats showed a highly significant increase in their thyroid content of MDA as compared to those of control and diabetic non-treated rats (p<0.001) (Tab. 2& Fig.3).The histopathological resultsHistopathological examination of the transverse sections of the thyroid gland of the control group (group I ) revealed that the gland is covered by thin connective tissue capsule which sent thin ill-defined septa divided the gland into small lobules each of which contains many follicles of variable sizes and shape, rounded or oval. At the periphery of the thyroid lobes, the larger thyroid follicles were mostly located. The follicles separated from each other by scanty connective tissue (Fig.1: C). Each follicle is surrounded by a thin basement membrane and lined by thyrocytes, follicular cells, which form a single layer of simple cuboidal epithelium with pale cytoplasm and basal or central nuclei. The lumina of the follicles were filled with a homogenous acidophilic substance called colloid (Fig.1: C 1). Also, the thyroid gland had the parafollicular cells, C cells, which always situates within the basement membrane or located adjacent to the thyroid follicles and reside in the connective tissue. These cells were small and have a dark stained nuclei compared with the follicular cells (Fig.3: C). A strong PAS positive reaction was seen in the basement membrane of the follicles and it appeared more prominent in the colloid (Fig. 2: C). By using toludine blue stain, two types of mast cells could be seen in the thyroid tissue, perifollicular mast cell which was represented near the thyrocytes and stromal mast cell which was represented in the stroma. Mast cells are oval or rounded in shape showing intense metachromasia masking their nuclei (Fig.2: C1).Immunohistochemical results of the control group showed that the expression of thyroid C-cells was mild calcitonin immunopositive staining which was localized exclusively to C-cells (Fig.4: C & C1). Furthermore, moderate caspase-3 immunopositive expression on the cells were appeared as brown cytoplasmic deposits in thyroid sections (Fig.5: C & C1).Examination of ultrathin sections of the thyroid gland of the control group revealed more details of the thyroid follicle. It was lined by one layer of cuboidal epithelium, thyrocytes, which has well define regular nucleus containing prominent nuclei and scattered chromatin. The cytoplasm contained well-flattened cisternae of rough endoplasmic reticulum and normal mitochondria. The apical surfaces showed small microvilli that projected into the follicular lumen which contains the colloid (Fig.6: C, C1 & C2 and Fig.7: C). Histopathological examination of the transverse sections of the thyroid gland of the diabetic rats without treatment (group II) revealed that the structural details of the glands were more or less similar to the control group ,whereas it covered by thin connective tissue capsule (Fig. 1:D). The follicle is surrounded by a thin basement membrane and lined by single layer of thyrocytes which were flat in comparison to the control which were cuboidal (Fig.1: D1and C1). Some follicular lumina contain minimal amount of colloid or peripheral vacuolations furthermore some follicle were devoid of colloid (Fig. 1: D1and Fig. 2: D). A moderate PAS positive reaction was seen in the basement membrane of the follicles and in the colloid in comparison to the strong reaction in the control group (Fig. 2:D & C). The two types of mast cells were seen similar to the control group (Fig.2 D1 and C1). Immunohistochemical results of the thyroid glands of group II were closed similar to the control group showed mild calcitonin immunopositive (Fig.4: D, D1, and C, C1) and moderate caspase-3 immunopositive cells (Fig.5: D, D1 and C, C1). Ultra structural study of the thyroid gland of the diabetic group without treatment confirmed that the thyroid follicles were closed similar to the control group except that the lining epithelium, thyrocytes, were flat (Fig.6: D, D1 and Fig.7:D). Furthermore, the morphometric results clarified the histological results whereas the area percent of colloid was significantly decreased (p<0.001) when compared to the control group. In addition there were no significant difference in the area percent of metachromaisa of mast cell, in calcitonin content and caspase 3 when compared to the control group (Tab. 3). Examination of transverse sections of the thyroid gland of the diabetic rat received therapeutic doses of Sitagliptin for 8weeks (group III) showed evidence of focal hyperplasia that is characterized by multiple layers of follicular epithelial cells protruding into the lumen (Fig.1:T1 and Fig.3: T). It also showed focal epithelial hypertrophy where the epithelial cells are tall cuboidal to columnar. Furthermore, some thyrocytes had pyknotic nuclei and vacuolated cytoplasm and the lumen contained exfoliated cells (Fig.3: T). Moreover some follicles had characters of apoptotic cells: a shrunk dense nucleus, an eosinophilic cytoplasm and an irregular outline. Moreover, the colloid appeared fill the entire lumen in most of the follicles with peripheral vacuolations (Fig.3: T). In addition, marked connective tissue deposition were noticed in the capsule and in-between the follicles with presence of large congested blood vessel (Fig.1: T, T1). A strong PAS positive reaction was seen in the basement membrane and in the colloid of many follicles (Fig.2: T). both perifollicular and stromal mast cells appeared larger than the control group with increase areas of metachromasia (Fig.2: T1 & C1).Immunohistochemical results of the thyroid gland of Sitagliptin treated group showed marked expression of calcitonin immunopositive staining of thyroid C-cells which appeared stronger than normal (Fig.4: T, T1 and C, C1). In addition, marked caspase-3 immunopositive cells were appeared as massive dense brown cytoplasmic deposits in thyroid sections when compared with the control group (Fig.5: T, T1 and C, C1).Morphometric results revealed that the means of area percent of the colloid and metachromasia were had highly significant increased (p<0.001) when compared to the control group. In addition to significant increase of the means of the area percent of calcitonin and caspase 3 when compared to the control (Tab. 3).Examination of ultrathin sections of the thyroid gland of the diabetic group treatment with sitglaptin revealed that the thyroid follicle lined by more than one layer of thyrocytes .Some thyrocytes contain irregular heterochromatic pyknotic nucleus and others have deep indentation. The cytoplasm contains; apical small microvilli that protruded into the follicular lumen, many cytoplasmic vacuoles, scanty mitochondria, dilated rough endoplasmic reticulum and dilated engorged Golgi apparatus with presence of many apical secretory vesicles. In addition there were presence of scattered primary lysosomes and many secondary lysosomes which contain electron dense apoptotic bodies (Fig.6: T, T1 and Fig.7: T, T1, and T2).
|
|
|
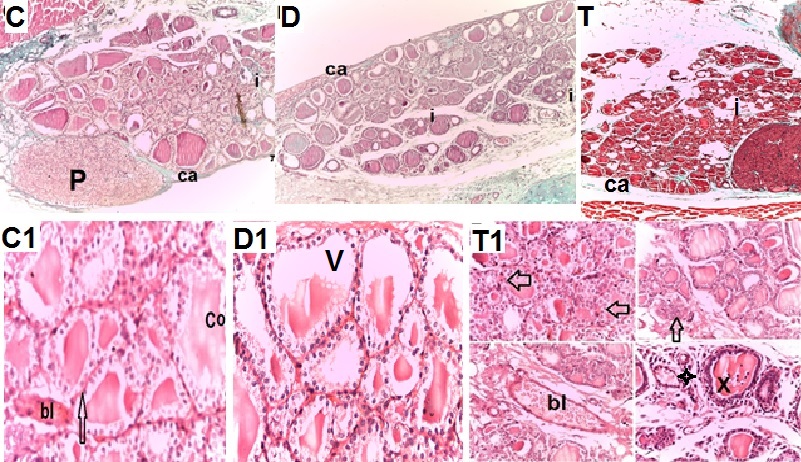 | Figure 1. Photomicrographs of transverse sections of the thyroid glands of the adult male rats of all studied groups demonstrating that: |
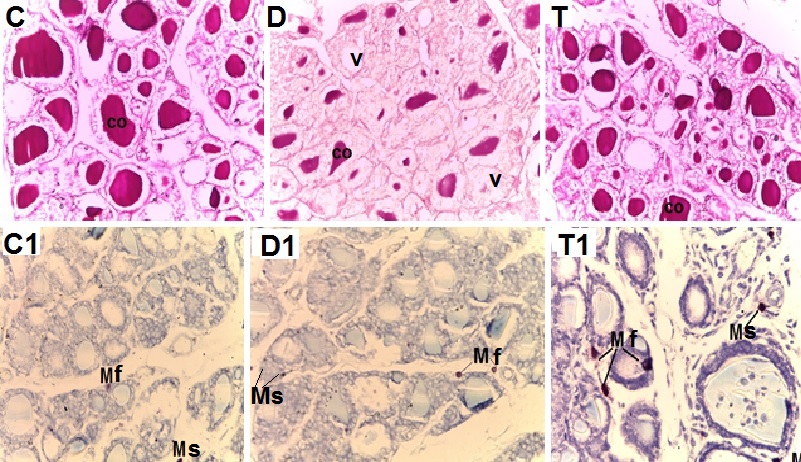 | Figure 2. Photomicrographs of transverse sections of the thyroid glands of the adult male rats of all studied groups demonstrating that: |
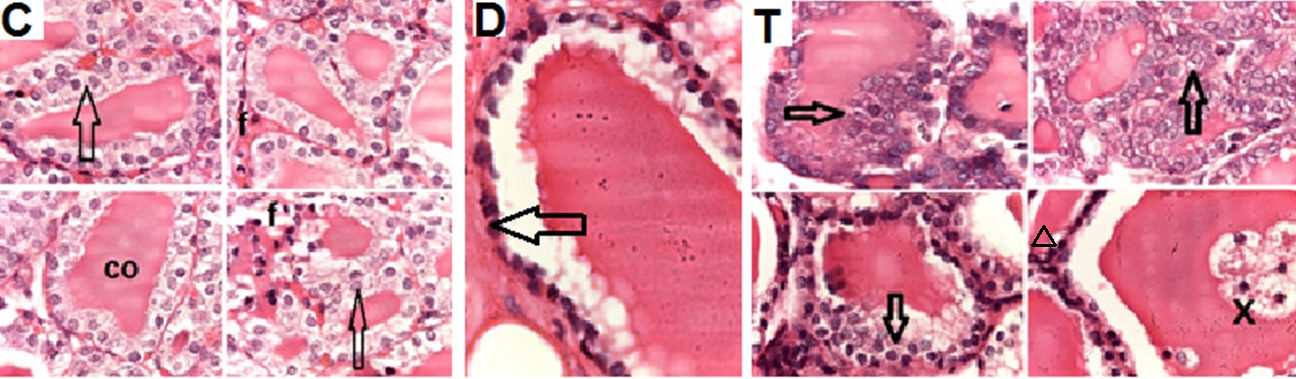 | Figure 3. Photomicrographs of high magnification of transverse sections of the thyroid glands of the adult male rats of all studied groups demonstrating that: |
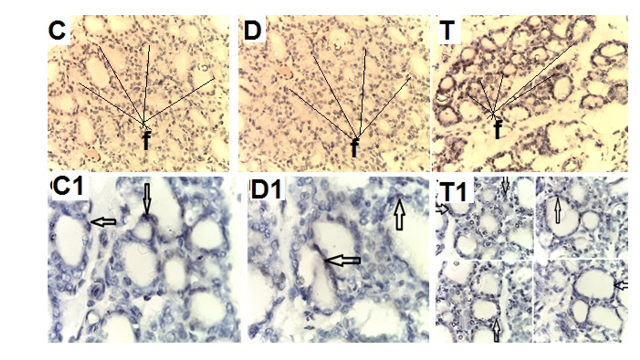 | Figure 4. Photomicrographs of transverse sections of the thyroid glands of the adult male rats of all studied groups demonstrating that: |
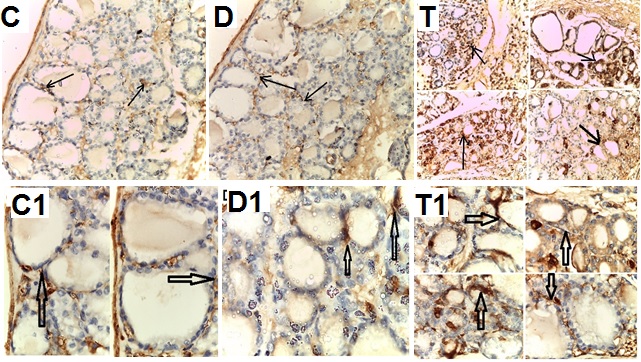 | Figure 5. Photomicrographs of transverse sections of the thyroid gland of the adult male rats of all studied groups demonstrating that: |
 | Figure 6. Electron micrographs of ultrathin sections of the thyroid glands of the adult male rats of all studied groups demonstrating that: |
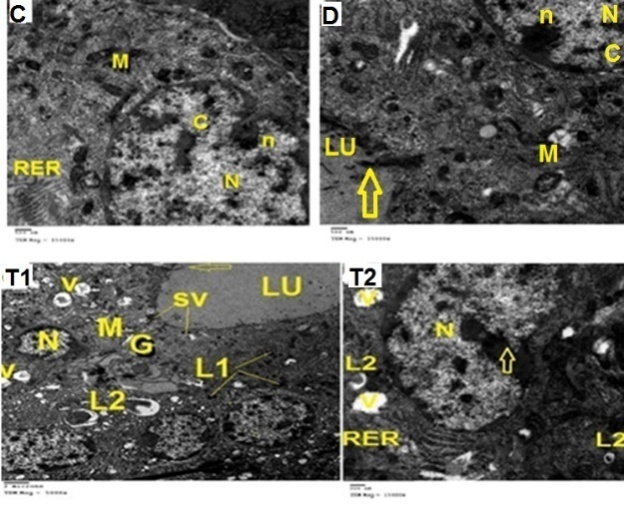 | Figure 7. Electron micrographs of ultrathin sections of the thyroid glands of the adult male rats of all studied groups demonstrating that: |
4. Discussion
- Biochemical study of group II (diabetic rats without treatment), showed evidences of hypothyroidism whereas there is decrease in the serum levels of the free T3 and T4 as well as thyroglobulin. The serum levels of the thyroid hormones seemed to reflect the histological changes in the thyroid gland; the follicles are lined by single layer of flat thyrocytes. Some follicular lumina contain minimal amount of colloid with peripheral vacuolations, furthermore some follicle were devoid of colloid, in addition to week PAS positive reaction in the basement membrane of the follicles and moderate reaction in the colloid in comparison to the strong reaction in the control group. The previous results indicate that diabetes mellitus without treatment lead to hypo function via disturbed an immune response or disturbed hormonal axe. Similar suggestion was reported by Baker (1992) who reported that autoimmune thyroiditis is often associated with other autoimmune diseases such as diabetes and Addison's disease, possibly suggesting a common defect predisposing to apoptotic destruction of the endocrine organs. Similar, Bestetti et al. (1987) and Donckier (2003) estimated the structures and hormonal secretion of the thyroid glands in adult male rats 2 month after streptozotocin injection (drug induced diabetes mellitus) without treatment revealed reduction of the hypothalamo-pituitary-thyroid axis, in addition to reduction in the intracolloidal thyroglobulin and T3 as well as intraepithelial thyroglobulin.Also, the results of the current study went hand in hand with the results of Norman and Litwack (1987) who stated that follicles of low epithelium indicated inactive thyroid. In addition, in the present study, most of the follicles contained small amount of colloid with many peripheral vacuolations due to hypo-activity of the gland similar observation was reported by Wheater et al. (1991) and Conde et al. (1991). Also, in consistence with the current study, Ugasio et al. (1991) showed that ecological factors influencing rat thyroid glands led to dystrophic changes in the form of decrease cell height, fusion of the follicles and desquamation of the epithelium into the follicular cavity.In the present study, in diabetic rats received therapeutic dose of Sitagliptin for 8weeks (group III) showed significant increase in free T3 serum level and thyroid content of MDA with decrease in the thyroid contents of the GSH, SOD and CAT activities, in addition to strong PAS positive reaction and significant increase of area percent of colloid. Also, there are evidences of focal hyperplasia and focal epithelial hypertrophy in association with enhancement of apoptosis which considered as a strong sign of proliferative changes, these changes might be owed to oxidative damage caused by Sitagliptin with generation of Reactive Oxygen Species (ROS) and lipid peroxidation. Similar suggestion was reported by Koehler and Drucker (2006) who cited that increased ductal cell turnover and ductal metaplasia may predispose to pancreatic cancer. Also, in the present study, congested and dilated interstitial blood vessels could be referred to oxidative stress and lipid peroxidation which might affect vascular walls leading to their dilatation and congestion. From the foregoing it is clear that Sitagliptin could seriously affect thyroid gland with subsequent disturbed hormones which play an important role in most of body physiological processes and can regulate and maintain normal physical, mental and sexual activities (Slotkin and Seidler, 2007 and Hong et al., 2009). Similar suggestion was reported by Hartoft et al. (2005) who cited that it is known that the size of a follicle depends on the amount of colloid. Giovannuccim et al. (2010) added, Sitagliptin showed increased pancreatic ductal hyperplasia in a small rodent model study. Similarly Stulc and Sedo (2010) reported that DPP-IV inhibitor has a complex role in relationship to cancer and may influence all stages of cancer from apoptosis, migration, invasion, and metastasis to even chemotherapy sensitivity. Similar concerns have been reported by Gier and Butler (2013) who observed raised risk for thyroid cancer with GLP-1 receptor agonist liraglutide. Elashoff et al. (2011); Arrebola et al. (2014) and Monami et al. (2014) reported that investigating GLP-1 based drugs that analyzed the US Food and Drug Administration’s (FDA) reported a six fold increased risk of pancreatic cancer, as well as an increased risk of thyroid cancer, in people treated with exenatide or sitagliptin (a DPP-4inhibitor). In the present study histological examination of thyroid mast cells in the diabetic rats treated by Sitagliptin revealed that the perifollicular and the stromal mast cells appeared larger and more abundant with significant increase area percent of metachromasia. This referred to the fact that mast cell population increased to overcome the damage induced by oxidative stress. Catini and Legnaioli (1992) reported that the thyroid mast cells which, appeared to be the regulator of local homeostasis and their condition reflected immune disturbances and other pathogenesis. Also, Gerbilsky et al. (1992) concluded that micro-environmental factors might influence the differentiation of the mast cell subtypes and the perifollicular mast cells might have a role in thyroid function and regulation. In addition, Kornilovskaya et al (1996) demonstrated similar changes in response to altrazine and suggested a functional heterogeneity within the rat thyroid mast cell populations. Similarly Toda et al. (2000) mentioned that the mast were suggested to release growth factor that modulates folliculogenesis and angiogenesis involved in the repair of thyroid tissue affected in sub-acute thyroiditis.Immunohistochemical results of the thyroid gland of Sitagliptin treated group showed marked expression of calcitonin immunopositive staining of thyroid C-cells with significant increase in the serum level of calcitonin hormone explain the fact that Sitagliptin cause proliferative changes in the C cells of the thyroid gland. Matveyenko et al. (2009) explained that DPP-4 inhibitors may also cause long-term hormonal alterations as it is considered as a potential endocrine disrupter. The most commonly affected endocrine gland is the thyroid as it is considered as a sensitive tissue leading to long-term effects on thyroid function. Also, Elisei et al. (2004); Costante et al. (2007) and Machens et al. (2009) mentioned that calcitonin is regarded as an important clinical biomarker for C-cell diseases such as medullary thyroid carcinoma and hereditary C-cell hyperplasia because of its high sensitivity and specificity. Moreover, because pancreatitis is a risk factor for pancreatic cancer, long-term GLP-1 receptor activation might lead to increased risk for pancreatic cancer (Rebours et al., 2009 and Singh et al., 2013). It has also been suggested that immune modulatory effects of DPP-4 inhibition might increase risk for all cancers (Matteucci and Giampietro, 2009 and Waser et al., 2011). The mode of action for C-cell tumorigenesis in rats and mice by long-acting GLP-1R agonists indicates that diffuse C-cell hyperplasia should precede focal C-cell hyperplasia as described for the actions of liraglutide in mice. In rodents Sitagliptin, GLP-1 receptor, stimulation induces C cell hyperplasia and medullary thyroid carcinoma. The effects of GLP-1 on C cells in humans remain to be established (Knudsen et al., 2010 and Frohlich et al., 2012). Diffuse C-cell hyperplasia is a physiological response due to chronic stimulation of calcitonin secretion. Focal C-cell hyperplasia is considered a preneoplastic finding. In humans, it has recently been reported that the Sitagliptin was consistently expressed in C-cells in neoplastic and hyperplastic lesions and in about one-third of normal C-cells in thyroids using immunofluorescence and immunohistochemistry (Gier et al., 2012). Recently, a similar caution was made by the FDA (Food and Drugs Administration’s) with regard to pancreatitis associated with Sitagliptin treatment (Engel et al., 2010; Engel et al., 2013 and Nelson et al., 2014). Also, thyroid tumors were reported to be more common in rodent toxicology studies with the GLP-1 agonist liraglutide, although the relevance of this in humans has been questioned (Stulc and Sedo, 2010 and Bonner-Weir et al., 2014).In the present study degeneration and apoptosis of follicular cells were noticed in Sitagliptin treated rats as evidenced histologically by presence of some pyknotic thyrocytes contained shrunken nuclei and vacuolated cytoplasm with presence of exfoliated cells in the lumina, in addition to the presence of multiple secondary lysosomes containing electron dens apoptotic bodies, moreover by immunohistochemical finding, a strong caspase 3 protein expression were present. Cohen, (1997) previously explained these finding by that Caspase-3 is a member of interlukein converting enzymes. It is the most commonly one involved in the execution of apoptosis in various cell types. Increase reactivity for caspase-3 could indicate cellular apoptosis. Jeong et al. (2006) and Yu et al. (2008) reported similar degeneration and apoptosis of follicular cells of treated rats exposed to oxidative stress by organophosphorus insecticide. In contrast Andrikoula and Tsatsoulis (2001) reported that activation of apoptosis may play an important role in thyroid homeostasis and autoimmune diseases. Because, in the current study there were increases in stratification of the follicular cells with strong immunopositive reaction of caspase 3, we can explain that the apoptosis is involved to maintain a proper balance in cell populations. Apoptosis eliminates cells in order to maintain a constant number of cells within a proliferating population, or to eliminate cells no longer needed. Similar suggestion were mentioned by Cohen and Eisenberg (1992); Fisher et al. (1995) and Dianzani et al. (1997) who reported that, to reduce the number of proliferated cells, death ligands on some of the immune cells bind to, and specifically activate, the death receptors on other immune cells. The result is that the activated immune cells eliminate each other by either apoptotic suicide or fratricide. Loss of the ability to reduce immune cell numbers through this mechanism results in unregulated expansion (hyperplasia) of immune cells leading to lymphoproliferative autoimmunity. Examples of this include a Lupus-like disease, in similar condition results in organ-specific autoimmunity, including thyroiditis. In a trial to connect the positive data in the present study, stratification of the thyrocytes, increase calcitonin hormonal level with increment of area percent of calcitonin in addition to increment of oxidative parameter with significant increase of mean area percent of caspase 3, we can confirm that the main cause of these pathological changes referred to the proved oxidative stress which caused by Sitagliptin. Similar suggestion was reported by Mc Conkey and Orrenius (1996) who reported that several lines of evidence support the involvement of ROS in many models of apoptotic cell death. Also, Sugawara et al. (2002) cited that Reactive oxygen species (ROS) generation and overload of cellular calcium Ca2 ions may be involved in the alteration of the thyroid morphology and the induction of caspase pathways in thyroid cells. Duchen (2000) explain the interactions among transporters, pumps, channels and binding proteins under pathological conditions of Ca2 overload, particularly in association with oxidative stress, may trigger pathological states that lead to apoptotic cell death.. Furthermore, Friedman et al. (2007) showed that reactive free radical stimulates plasma membrane NADH oxidase in mammalian cells and causes production of ROS. Also, Esmekaya et al., 2010 reported that reactive oxygen species (ROS) generation and overload of cellular calcium [Ca2 ] ions may be involved in the alteration of thyroid morphology and the induction of caspase pathways in thyroid cells. Also, in vitro and in vivo experiments by Kurebayashi et al. (2014) had demonstrated that long-term GLP-1 receptor activation is associated with increased calcitonin gene transcription and subsequently with C-cell proliferation and tumor formation in both rats and mice. Because millions of patients will take DPP-IV inhibitors for life, there is some urgency to more fully understand the long-term risks associated with DPP-IV inhibition. From our study, we conclude that Sitagliptin when given to diabetic rats over prolonged period will cause alternation of the thyroid hormones with follicular and parafolliclular cellular changes with focal hypertrophic changes. So, we recommended that, before starting and during Sitagliptin treatment, monitoring of thyroid functions have to be assessed. Also, more researches must be done to find tight safety methods to achieve a glycemic control.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML